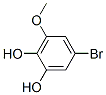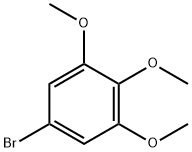
1-Bromo-3,4,5-trimethoxybenzene synthesis
- Product Name:1-Bromo-3,4,5-trimethoxybenzene
- CAS Number:2675-79-8
- Molecular formula:C9H11BrO3
- Molecular Weight:247.09
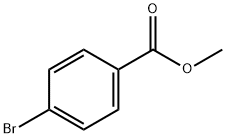
619-42-1
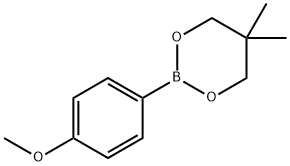
213596-33-9

2675-79-8
The general procedure for the synthesis of 1-bromo-3,4,5-trimethoxybenzene from methyl p-bromobenzoate and 2-(4-methoxyphenyl)-5,5-dimethyl-1,3,2-dioxaborole cyclohexane is as follows: aryl halide (0.67 mmol, 1.0 eq.), aryl boronate (0.81 mmol, 1.2 eq.) were added to a Schlenk tube in the following order, potassium phosphate or CsF (3.0 eq.), Pd catalyst (0.1 eq.) and 2-(dicyclohexylphosphino)biphenyl (0.2 eq.). When using Pd(OAc)2 as the catalyst, CsF is required as the base (see Table 8, entries 13-15). Subsequently, a PTFE-coated stirrer was added to the reaction system and the reaction was carried out at room temperature. The reaction mixture was degassed under high vacuum three times for 10 min each and backfilled with nitrogen. Anhydrous dioxane was added through the T-neck and the reaction mixture was heated to 110°C and kept for 18 hours. Upon completion of the reaction, the mixture was cooled to room temperature and diluted with dichloromethane (10 mL). The reaction solution was filtered and the filtrate was washed with dichloromethane (100 mL). Finally, the filtrate was concentrated and purified by silica gel column chromatography.
Yield:2675-79-8 1256 g
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene at 90;Large scale;Reagent/catalyst;
Steps:
1.3; 2.3; 3.3; 4.3; 5.3; 6.3 Synthesis of S3, 5-bromo-1,2,3-trimethoxybenzene (1)
Dissolve 1256 grams of Y-3 in 5.0 liters of dimethyl carbonate,1745 grams of DBU was added to the system, the system was naturally heated to 8-10 degrees, the oil bath was heated to 90 degrees and refluxed overnight, and the reaction was monitored by TLC until the reaction was complete. The reaction system was cooled to room temperature, and the reaction solution was concentrated to recover dimethyl carbonate. The concentrated reaction solution was poured into 5 liters of ethyl acetate and 2 liters of water. After stirring for 30 minutes, the layers were separated. The water layer was to be treated and the organic layer was used 1 liter in turn Wash twice with 2N HCl, twice with 1L 2N NaOH, and once with 1L water, and set aside the organic layer. The aqueous layer of the reaction solution, the acid aqueous layer, the alkaline aqueous layer, and the water-washed layer were extracted twice with 1 liter of ethyl acetate. The organic layers were combined, washed with 1L of saturated brine, dried with anhydrous sodium sulfate, and decolorized by adding activated carbon. It was filtered and spin-dried to obtain 1256 grams of crude product, which was recrystallized with ethanol-petroleum ether mixed solvent to obtain the pure product of target 1.
References:
CN113563163,2021,A Location in patent:Paragraph 0038; 0043-0054
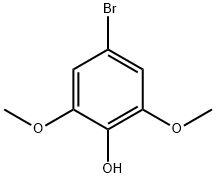
70654-71-6
21 suppliers
$570.00/1g

77-78-1
319 suppliers
$22.00/25g

2675-79-8
231 suppliers
$8.00/1g
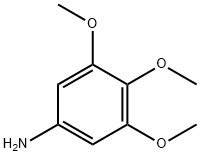
24313-88-0
289 suppliers
$10.00/1g

2675-79-8
231 suppliers
$8.00/1g

619-42-1
373 suppliers
$7.00/10g

213596-33-9
31 suppliers
$79.00/1g

2675-79-8
231 suppliers
$8.00/1g
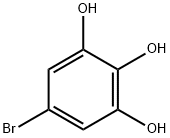
16492-75-4
57 suppliers
$62.00/100mg

2675-79-8
231 suppliers
$8.00/1g