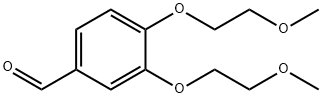
3,4-bis(2-Methoxyethoxy)benzaldehyde synthesis
- Product Name:3,4-bis(2-Methoxyethoxy)benzaldehyde
- CAS Number:80407-64-3
- Molecular formula:C13H18O5
- Molecular Weight:254.28

627-42-9
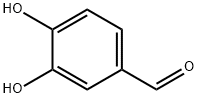
139-85-5

80407-64-3
The general procedure for the synthesis of 3,4-bis(2-methoxyethoxy)benzaldehyde from 2-chloroethyl methyl ether and 3,4-dihydroxybenzaldehyde: 121 g (1 eq.) of 3,4-dihydroxybenzaldehyde was dissolved in 1200 ml of dimethylformamide (DMF) and stirred until a clarified solution formed; 323 g (4 eq.) of 2-chloroethyl methyl ether, 944 g (8 eq.) of anhydrous potassium carbonate, and 23.6 g of tetrabutyl ammonium bromide were added in turn. ) anhydrous potassium carbonate and 23.6 g of tetrabutyl ammonium bromide; the reaction system was purged with nitrogen for three times, and then the reaction mixture was heated to 105?110°C under nitrogen protection, and the reaction was carried out for 13?14 h. After the reaction was completed, the reaction mixture was filtered, and the filtrate cake was rinsed with dichloromethane (DCM) until the filtrate was colorless; the filtrates were combined, and the reaction mixture was concentrated under reduced pressure until no fractions were evaporated; the residue was added with DCM and cooled to room temperature. DCM, cooled to room temperature, washed sequentially with 3N potassium hydroxide solution, water and brine, the organic phase was dried with anhydrous sodium sulfate, and concentrated under reduced pressure to 45 ℃ to obtain 210 g of the oily product 3,4-bis(2-methoxyethoxy)benzaldehyde in 96.4% yield.

6482-24-2
296 suppliers
$9.00/1g

139-85-5
849 suppliers
$5.00/1g

80407-64-3
63 suppliers
$6.00/100mg
Yield:80407-64-3 98%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 100;
References:
Chandregowda, Venkateshappa;Venkateswara Rao, Gudapati;Chandrasekara Reddy, Goukanapalli [Heterocycles,2007,vol. 71,# 1,p. 39 - 48]

4296-15-5
63 suppliers
$27.00/100mg

139-85-5
849 suppliers
$5.00/1g

80407-64-3
63 suppliers
$6.00/100mg
36865-41-5
227 suppliers
$7.00/10g

139-85-5
849 suppliers
$5.00/1g

80407-64-3
63 suppliers
$6.00/100mg
121-33-5
1188 suppliers
$9.00/1g

80407-64-3
63 suppliers
$6.00/100mg