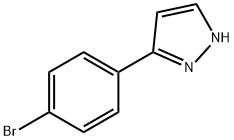
3-(4-BROMOPHENYL)-1H-PYRAZOLE, 97% synthesis
- Product Name:3-(4-BROMOPHENYL)-1H-PYRAZOLE, 97%
- CAS Number:73387-46-9
- Molecular formula:C9H7BrN2
- Molecular Weight:223.07
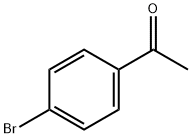
99-90-1
477 suppliers
$6.00/25g
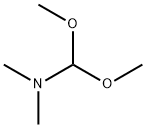
4637-24-5
646 suppliers
$5.00/25g

73387-46-9
113 suppliers
$21.50/1g
Yield: 80%
Reaction Conditions:
Stage #1:para-bromoacetophenone;N,N-dimethyl-formamide dimethyl acetal with N,N-dimethyl-formamide at 80;
Stage #2: with hydrazine hydrate in ethanol at 80; for 2 h;
Steps:
1 (1)
(1)
3-(4-bromophenyl)-1H-pyrazole
5 g (25.0 mmol) of 4-bromoacetophenone and 3.59 g (30.1 mmol) of 1,1-dimethoxy-N,N-dimethylmethanamine were added to 40 ml of DMF.
The mixture was heated to 80° C. for overnight.
After cooling, the mixture was poured to water (150 mL) and extracted with EA (100 mL*3).
The combined organic phase was washed with brine, concentrated to give red liquid (6 g).
The thus obtained product was redissolved in 50 ml of ethanol and treated with hydrazine monohydrate (3.5 ml, 75 mmol).
After the reaction was stirred at 80° C. for 2 h, it was cooled to 23° C. and poured to ice-water.
Solid was precipitated out of the solution and filtered, washed with water, and dried to give 3.6 g of compound 3-(4-bromophenyl)-1H-pyrazole as yellow solid (yield: 80%).
1H NMR (400 MHz, CDCl3): δ=7.58-7.62 (m, 3H), 7.49-7.51 (m, 2H), 6.58 (d, J=2.4, 1H).
References:
GENESTE, Hervé;TURNER, Sean Colm;OCHSE, Michael;DRESCHER, Karla;BLACK, Lawrence A.;JANTOS, Katja US2014/148461, 2014, A1 Location in patent:Paragraph 0341-0342
37614-58-7
37 suppliers
$87.00/250mg

73387-46-9
113 suppliers
$21.50/1g

73387-60-7
26 suppliers
$30.50/10g

73387-46-9
113 suppliers
$21.50/1g

99-90-1
477 suppliers
$6.00/25g

73387-46-9
113 suppliers
$21.50/1g
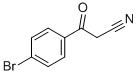
4592-94-3
168 suppliers
$6.00/250mg

73387-46-9
113 suppliers
$21.50/1g