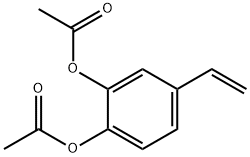
3,4-diacetoxystyrene synthesis
- Product Name:3,4-diacetoxystyrene
- CAS Number:57142-64-0
- Molecular formula:C12H12O4
- Molecular Weight:220.22
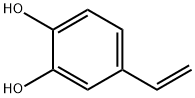
6053-02-7
57 suppliers
$260.00/10mg

75-36-5
589 suppliers
$17.92/100G

57142-64-0
50 suppliers
inquiry
Yield:57142-64-0 87.3%
Reaction Conditions:
with 10H-phenothiazine;triethylamine in tert-butyl methyl ether at -5 - 20; for 1 h;Green chemistry;
Steps:
2 Example 2
Add 3,4-dihydroxystyrene (136 g) to a 2 L four-neck bottle. triethylamine (212g), phenothiazine (1.4g), methyl tert-butyl ether (544g), dry ice-ethanol bath to -5 to 0 °C, acetyl chloride (168 g) was added dropwise with stirring, and the internal temperature was controlled at -5 to 0 °C. After the completion of the dropwise addition, the temperature was raised at 10 to 20 °C to continue the reaction for 1 hour. sampling analysis (central control 1, raw material After completion of the reaction, the mixture was filtered, and the cake was washed with methyl t-butyl ether (50 g × 3). The filtrate was quenched by the addition of 8 g of methanol, and the reaction was stirred for 10 minutes. After completion, phenothiazine (1.4 g) was added, and the reaction liquid was concentrated, and methyl tert-butyl ether (580 g) was collected to obtain crude product (220 g). Crude recrystallized at low temperature the product 3,4-diacetoxystyrene (192 g) was obtained in a yield of 87.3%, and was analyzed by sampling (main content >97%).
References:
CN108911982,2018,A Location in patent:Paragraph 0046; 0047

108-24-7
0 suppliers
$14.00/250ML

57142-64-0
50 suppliers
inquiry

6053-02-7
57 suppliers
$260.00/10mg

108-24-7
0 suppliers
$14.00/250ML

57142-64-0
50 suppliers
inquiry
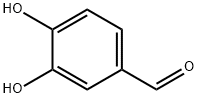
139-85-5
851 suppliers
$5.00/1g

57142-64-0
50 suppliers
inquiry