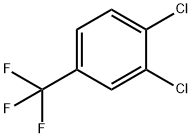
3,4-Dichlorobenzotrifluoride synthesis
- Product Name:3,4-Dichlorobenzotrifluoride
- CAS Number:328-84-7
- Molecular formula:C7H3Cl2F3
- Molecular Weight:215
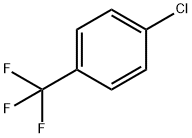
98-56-6
410 suppliers
$11.00/25g

328-84-7
385 suppliers
$5.00/5g
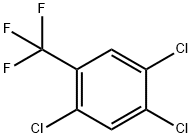
56148-83-5
16 suppliers
inquiry
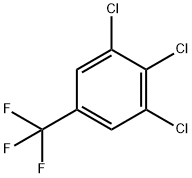
50594-82-6
244 suppliers
$6.00/5g
Yield:328-84-7 82.46% ,50594-82-6 5.25% ,56148-83-5 12.29%
Reaction Conditions:
with sulfuric acid;chlorine;aluminum (III) chloride;iron at 110;Product distribution / selectivity;
Steps:
1
Into a 1000 ml 4-neck flask add p-chloro trifluoromethyl benzene 1000 g, metal powdered iron 6 g, and anhydrous aluminum chloride 10 g, start agitation, heat the mixed solution to 100° C., then slowly feed concentrated sulfuric acid-dried chlorine 1028 g under reaction temperature 110° C., take sample, make GC analysis and trace to judge the reaction end point, lower the temperature, discharge the products, and obtain mixed solution of chlorides. The mixed solution contains 82.46% the 3,4-dichlorotrifluoromethyl benzene, 5.25% the 3,4,5-trichloro trifluoromethyl benzene and 12.29% 2,4,5-trichloro trifluoromethyl benzene and polychlorides.
References:
Zhejiang Weihua Chemical Co., Ltd. US2009/240083, 2009, A1 Location in patent:Page/Page column 3
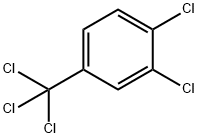
13014-24-9
123 suppliers
$15.00/1g

328-84-7
385 suppliers
$5.00/5g
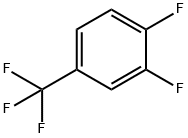
32137-19-2
211 suppliers
$8.00/1g
584-08-7
1252 suppliers
$5.00/100g

328-84-7
385 suppliers
$5.00/5g
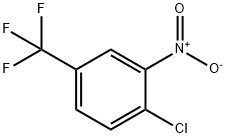
121-17-5
32 suppliers
$19.00/250g

328-84-7
385 suppliers
$5.00/5g
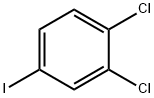
20555-91-3
193 suppliers
$8.00/5g
![4-[2,2,2-Trifluoro-1-[(trimethylsilyl)oxy]ethyl]morpholine](/CAS/20200401/GIF/289706-46-3.gif)
289706-46-3
18 suppliers
$45.00/10mg

328-84-7
385 suppliers
$5.00/5g