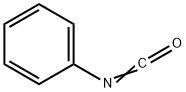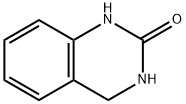
3,4-DIHYDROQUINAZOLIN-2(1H)-ONE synthesis
- Product Name:3,4-DIHYDROQUINAZOLIN-2(1H)-ONE
- CAS Number:66655-67-2
- Molecular formula:C8H8N2O
- Molecular Weight:148.16
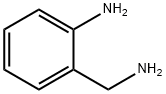
4403-69-4
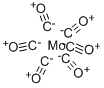
13939-06-5

66655-67-2
The general procedure for the synthesis of 3,4-dihydro-1H-quinazolin-2-one from 2-aminobenzylamine and molybdenum hexacarbonyl was as follows: the reaction was carried out in a two-chamber system. First, 2-aminobenzylamine, Pd(OAc)2 (0.05 eq.) and Cu(OAc)2 (0.5 eq.) were added to the reaction chamber and dissolved in 1,4-dioxane (2 mL). In a CO chamber, Mo(CO)6 (200 mg) was dissolved in 1,4-dioxane (2 mL). Subsequently, 1,8-diazabicyclo[5.4.0]undec-7-ene was added to the system. The two-compartment system was placed in a Dry-Syn heating block and heated to 95°C for the reaction. Upon completion of the reaction, the reaction mixture was filtered through a short silica gel plug and subsequently purified by fast column chromatography.
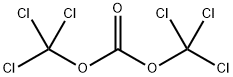
32315-10-9
441 suppliers
$10.00/1g

4403-69-4
112 suppliers
$9.00/1g

66655-67-2
53 suppliers
$45.00/10mg
Yield: 99%
Reaction Conditions:
in tetrahydrofuranInert atmosphere;
Steps:
3,4-Dihydroquinazolin-2(1H)-one (10b)
Triphosgene (3.31 g, 12.3 mmol) was added toa solution of 2-(aminomethyl)aniline (1.50 g, 12.3 mmol) in 30 ml THF under a nitrogenatmosphere. After precipitation of a colorless solid, stirring was continued for 30 minbefore water was added. The solution was extracted several times with ethyl acetate. Thecombined organic layers were washed with brine and dried over anhydrous MgSO4 toafford 10b (1.80 g, 12.2 mmol, 99 %) as colorless solid. 1H-NMR (500 MHz, DMSO-d6):δ = 4.30 (s, 2H), 6.75-6.77 (m, 2H), 6.84 (ddd, 3J = 3J = 7.4 Hz, 4J = 1.9 Hz, 1H), 7.06 (d,3J = 7.3 Hz, 1H), 7.09 (dd, 3J = 3J = 7.7 Hz, 1H), 8.98 (s, 1H). 13C-NMR (125 MHz,DMSO-d6): δ = 42.4, 113.4, 118.0, 120.8, 125.5, 127.4, 138.0, 154.5.
References:
Grombein, Cornelia M.;Hu, Qingzhong;Rau, Sabrina;Zimmer, Christina;Hartmann, Rolf W. [European Journal of Medicinal Chemistry,2015,vol. 90,p. 788 - 796] Location in patent:supporting information
201230-82-2
1 suppliers
inquiry

4403-69-4
112 suppliers
$9.00/1g

66655-67-2
53 suppliers
$45.00/10mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

66655-67-2
53 suppliers
$45.00/10mg

124-38-9
134 suppliers
$175.00/23402

4403-69-4
112 suppliers
$9.00/1g

66655-67-2
53 suppliers
$45.00/10mg