3,4-Dimethyl-2(1H)-quinolinone synthesis
- Product Name:3,4-Dimethyl-2(1H)-quinolinone
- CAS Number:17336-90-2
- Molecular formula:
- Molecular Weight:0
Yield:17336-90-2 86%
Reaction Conditions:
with triethylamine in dichloromethane at 60; for 12 h;Inert atmosphere;Glovebox;Schlenk technique;
Steps:
General Procedure of Products (2).
General procedure: An oven-dried Schlenk tube (25 mL) containing a stirring bar was charged with the substrate (0.3 mmol). The Schlenk tube was then introduced in a glovebox where it was charged with triphosgene (44.5 mg, 0.15 mmol, 0.5 equiv.). The tube was taken out of the glovebox and connected to a vacuum line where it was evacuated and back-filled under N2 flow for at least 3 times. The DCM (5 mL) and Et3N (0.3 mmol, 1 equiv.) were added under N2 flow. Once added, the tube was closed at atmospheric pressure of N2 (1 atm) and stirred for 12 h at 60 °C. Then, the mixture was cooled to room temperature, quenched with 2 mL water, then concentrated in vacuo. The residue was purified by silica gel flash chromatography (petroleum ether/AcOEt 3/1) to give the pure target product 2. (the procedure of control experiments and the reaction in lager scale are similar.)
References:
Du, Guizhi;Wang, Zixiao;Zhang, Zhen [Heterocycles,2020,vol. 100,# 4,p. 600 - 608]

30931-93-2
0 suppliers
inquiry

17336-90-2
2 suppliers
inquiry
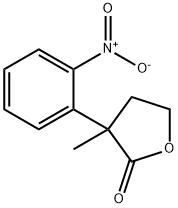
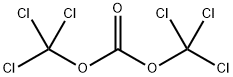
88426-92-0
0 suppliers
inquiry

17336-90-2
2 suppliers
inquiry
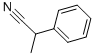
1823-91-2
136 suppliers
$20.00/5g

17336-90-2
2 suppliers
inquiry