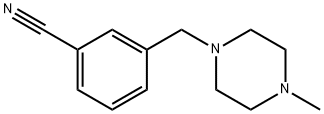
3-[(4-METHYLPIPERAZIN-1-YL)METHYL]BENZONITRILE synthesis
- Product Name:3-[(4-METHYLPIPERAZIN-1-YL)METHYL]BENZONITRILE
- CAS Number:859850-90-1
- Molecular formula:C13H17N3
- Molecular Weight:215.29
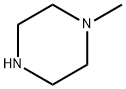
109-01-3
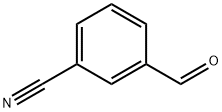
24964-64-5
![3-[(4-METHYLPIPERAZIN-1-YL)METHYL]BENZONITRILE](/CAS/GIF/859850-90-1.gif)
859850-90-1
General procedure for the synthesis of 3-((4-methylpiperazin-1-yl)methyl)benzonitrile from N-methylpiperazine and 3-cyanobenzaldehyde: Step 1: 3-Formylbenzonitrile (1.5 g, 11.45 mmol), N-methylpiperazine (1.49 g, 14.9 mmol) and sodium triacetoxyborohydride (3.63 g, 17.18 mmol) were dissolved in dichloromethane (75 ml), acetic acid (0.851 ml, 14.9 mmol) was added and the reaction was stirred at room temperature overnight. After completion of the reaction, the reaction mixture was diluted with dichloromethane and washed with 1 M sodium carbonate solution. The organic phase was dried with anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was purified by column chromatography (eluent: dichloromethane/methanol/ammonia = 97:3:0.5) to give 3-(4-methylpiperazin-1-ylmethyl)benzonitrile 1.7 g in 70% yield.
Yield:859850-90-1 70%
Reaction Conditions:
Stage #1: 1-methyl-piperazine;3-Cyanobenzaldehydewith sodium tris(acetoxy)borohydride;acetic acid in dichloromethane at 20;
Stage #2: with sodium carbonate in dichloromethane;water;
Steps:
14.A
Preparation 14: 1-[3-(4-WlethyI-piperazin-1-ylmethyl)-phenyI]-ethanone; STEP A; A mixture of 3-formyl-benzonitrile (1.5 g, 11.45 mmol), N-methyl piperazine (1.49 g, 14.9 mmol) and NaBH(OAc)3 (3.63 g, 17.18 mmol) in DCM (75 ml) and CH3COOH (0.851 ml, 14.9 mmol) was stirred overnight at room temperature, then diluted with DCM and washed with 1 M Na2CO3. The organic phase was dried over Na2SO4 and evaporated in vacuo. The crude mixture was purified by column chromatography (eluent: DCM/MeOH/NH4OH 97:3:0.5) to give 1.7 g of 3-(4- methyl-piperazin-1-ylmethyl)-benzonitrile. Y= 70%
References:
WO2007/113249,2007,A2 Location in patent:Page/Page column 60-61
