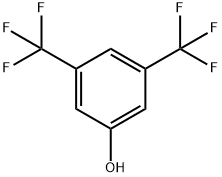
3,5-Bis(trifluoromethyl)phenol synthesis
- Product Name:3,5-Bis(trifluoromethyl)phenol
- CAS Number:349-58-6
- Molecular formula:C8H4F6O
- Molecular Weight:230.11
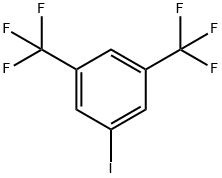
328-73-4

349-58-6
General procedure for the synthesis of 3,5-bis(trifluoromethyl)iodobenzene from 3,5-bis(trifluoromethyl)iodobenzene: In a test tube equipped with a magnetic stirrer, 3,5-bis(trifluoromethyl)iodobenzene (1.0 mmol), CuCl2 (13.4 mg, 0.1 mmol), KOH (336 mg, 6.0 mmol), ethylene glycol (12 μL, 0.2 mmol), and DMSO/H2O mixed solvent (1.0 mL/0.5 mL). The system was replaced by argon gas and then placed in an oil bath preheated to 120 °C with stirring for 24 hours. After completion of the reaction, it was cooled to room temperature and the reaction mixture was extracted by partitioning with 5% HCl aqueous solution and ethyl acetate. The organic phase was washed sequentially with water and saturated saline, dried over anhydrous MgSO4 and concentrated under reduced pressure to remove the solvent. The crude product was further purified by column chromatography (eluent: EtOAc/hexane) to afford the target product 3,5-bis(trifluoromethyl)phenol.

328-73-4
195 suppliers
$9.00/1g

349-58-6
183 suppliers
$11.00/1g
Yield:349-58-6 96%
Reaction Conditions:
with water;ethylene glycol;potassium hydroxide;copper dichloride in dimethyl sulfoxide at 120; for 24 h;Inert atmosphere;
Steps:
General Procedure A.
General procedure: To a test tube containing a magnetic bar was added aryl halide (1.0 mmol), CuCl2 (13.4 mg, 0.1 mmol), KOH (336 mg, 6.0 mmol), ethylene glycol(12 μL, 0.2 mmol), and DMSO/H2O (1.0 mL/0.5 mL). After flushing with argon, the mixture was stirred in a preheated oil bath at 120 °C for 24 h. After cooled to ambient temperature, the reaction mixture was distributed in aqueous HCl (5 %) and ethyl acetate. The organic layer was washed with water and brine, dried over anhydrous MgSO4, and concentrated under vacuum. The crude product was further purified by column chromatography (EtOAc/n-Hexane) to provide the phenols.
References:
Kim, Jihye;Battsengel, Oyunsaikhan;Liu, Yajun;Chae, Junghyun [Bulletin of the Korean Chemical Society,2015,vol. 36,# 12,p. 2833 - 2840] Location in patent:supporting information
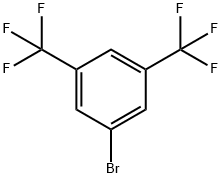
328-70-1
438 suppliers
$10.00/25 g

349-58-6
183 suppliers
$11.00/1g
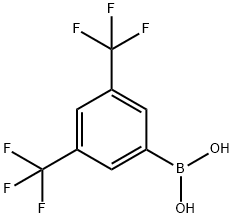
73852-19-4
297 suppliers
$5.00/1g

349-58-6
183 suppliers
$11.00/1g
30071-93-3
384 suppliers
$10.00/10 g

349-58-6
183 suppliers
$11.00/1g
![2-[3,5-BIS(TRIFLUOROMETHYL)PHENOXY]ETHANOL](/StructureFile/ChemBookStructure7/GIF/CB4780863.gif)
887268-12-4
13 suppliers
$164.00/500mg

349-58-6
183 suppliers
$11.00/1g