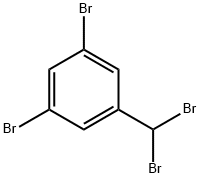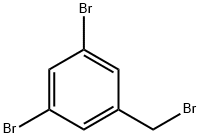
3,5-Dibromobenzyl bromide synthesis
- Product Name:3,5-Dibromobenzyl bromide
- CAS Number:56908-88-4
- Molecular formula:C7H5Br3
- Molecular Weight:328.83
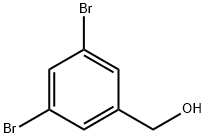
145691-59-4

56908-88-4
Under argon protection, (3,5-dibromophenyl)methanol (5.00 g, 0.021 mol) was dissolved in anhydrous tetrahydrofuran (250 mL), and the solution was cooled to 0 °C. Lithium aluminum hydride (1.62 g, 0.043 mol) was added slowly and in batches, followed by stirring the reaction mixture at room temperature overnight. Upon completion of the reaction, it was carefully quenched with water, ether (100 mL) was added and acidified with concentrated hydrochloric acid solution until the solid residue was completely dissolved. The reaction mixture was extracted with ether, the organic phases were combined and dried over anhydrous magnesium sulfate. After filtration, the organic phase was concentrated under reduced pressure to give 4.31 g of the target product. The structure of the product was confirmed by nuclear magnetic resonance hydrogen spectroscopy (1H NMR, CDCl3): δ 1.34 (s, 18H, tert-butyl hydrogen), 4.70 (s, 2H, benzyl hydrogen), 7.23 (d, 2H, J = 1.8 Hz, aromatic hydrogens), and 7.38 (t, 1H, J = 1.8 Hz, aromatic hydrogens). The crude alcohol (4.25 g) was dissolved in anhydrous dichloromethane (500 mL) and cooled at 0 °C. Triphenylphosphine (10.23 g, 0.039 mol) and carbon tetrabromide (12.93 g, 0.039 mol) were added sequentially, and the reaction mixture was stirred at room temperature for 1 hour. After completion of the reaction, the reaction was quenched with water and extracted with dichloromethane. The organic phase was dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The product was purified by fast column chromatography on silica gel using hexane as eluent to give 4.62 g (86% yield) of 1,3-dibromo-5-(bromomethyl)benzene.
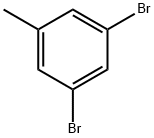
1611-92-3
316 suppliers
$6.00/5g

56908-88-4
153 suppliers
$6.00/1g
Yield:56908-88-4 74%
Reaction Conditions:
with NBS;dibenzoyl peroxide in Carbon tetrachloride; for 24 h;Reflux;
Steps:
1.c synthesis of Intermediate C
1,3-dibromo-5-toluene (4.96 g, 20 mmol) was dissolved in 150 ml of tetrachloromethane and then benzoyl peroxide (0.36 g, 1.5 mmol) and N-bromosuccinimide (3.74 g, 21 mmol) was added thereto, and the reaction mixture was heated under reflux for 24 hours. The reaction solution was then poured into water and extracted with methylene chloride. The organic compound was dried over magnesium sulfate and treated with dichloromethane / petroleum ether (v / v = 2: 3) was chromatographed on silica gel as a eluent, dried and steamed to give intermediate C (4.87 g, 74% yield) as a white solid.
References:
CN106946777,2017,A Location in patent:Paragraph 0060-0062

145691-59-4
120 suppliers
$6.00/100mg

56908-88-4
153 suppliers
$6.00/1g
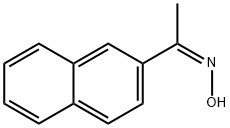
100485-60-7
0 suppliers
inquiry

56908-88-4
153 suppliers
$6.00/1g