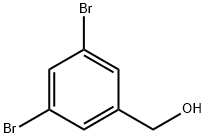
3,5-DIBROMOBENZYL ALCOHOL synthesis
- Product Name:3,5-DIBROMOBENZYL ALCOHOL
- CAS Number:145691-59-4
- Molecular formula:C7H6Br2O
- Molecular Weight:265.93
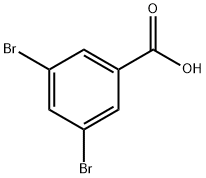
618-58-6

145691-59-4
Step 1: To a stirred solution of 3,5-dibromobenzoic acid (2 g, 7.168 mmol) in tetrahydrofuran (20 mL) was slowly added borane dimethyl sulfide (3.4 mL, 35.842 mmol, 5 eq.) at 0 °C. The reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, the mixture was cooled to 0 °C and the reaction was carefully quenched with methanol (appropriate amount). Subsequently, the reaction mixture was concentrated under reduced pressure to afford (3,5-dibromophenyl)methanol as an off-white solid (1.8 g, 94% yield). The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 4.47 (d, J = 6Hz, 2H), 5.39 (t, J = 6Hz, 1H), 7.49 (s, 2H), 7.65 (s, 1H).

618-58-6
274 suppliers
$6.00/5g

145691-59-4
121 suppliers
$6.00/100mg
Yield:145691-59-4 94%
Reaction Conditions:
with dimethylsulfide borane complex in tetrahydrofuran at 0 - 20;
Steps:
Step 1
Step 1 : To a stirred solution of 3,5-dibromobenzoic acid (2 g, 7.168 mmol) in tetrahydrofuran (20 mL) was added borane dimethylsulfide (3.4 mL, 35.842 mmol, 5 eq.) at 0 °C, and the reaction mixture was stirred at room temperature for overnight. The reaction mixture was quenched with methanol at 0 °C and concentrated under vacuo to obtain (3,5- dibromophenyl)methanol as an off white solid (1.8 g, 94%). H NMR (400 MHz, DMSO-d6) δ ppm 4.47 (d, J = 6 Hz, 2H), 5.39 (t, J = 6 Hz, 1 H), 7.49 (s, 2H), 7.65 (s, 1 H).
References:
WO2017/46739,2017,A1 Location in patent:Page/Page column 60
100485-60-7
0 suppliers
inquiry

145691-59-4
121 suppliers
$6.00/100mg
67973-33-5
23 suppliers
$661.00/1g

145691-59-4
121 suppliers
$6.00/100mg
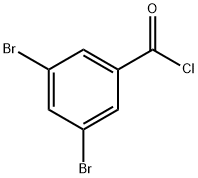
23950-59-6
18 suppliers
$74.23/1gm:
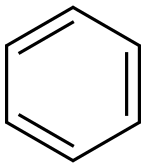
71-43-2
722 suppliers
$11.19/25ML
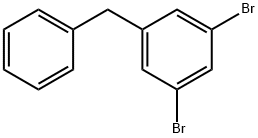
71572-36-6
4 suppliers
inquiry

145691-59-4
121 suppliers
$6.00/100mg
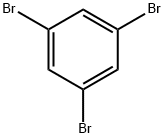
626-39-1
480 suppliers
$5.00/5g

145691-59-4
121 suppliers
$6.00/100mg