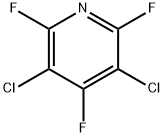
3,5-Dichloro-2,4,6-trifluoropyridine synthesis
- Product Name:3,5-Dichloro-2,4,6-trifluoropyridine
- CAS Number:1737-93-5
- Molecular formula:C5Cl2F3N
- Molecular Weight:201.96
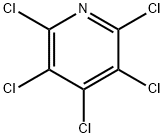
2176-62-7

1737-93-5
The general procedure for the synthesis of 3,5-dichloro-2,4,6-trifluoropyridine from pentachloropyridine (2,3,4,5,6-pentachloropyridine) was as follows: in a 1000mL three-necked flask, 600mL of DMI (1,3-dimethyl-2-imidazolidinone) and 146.72g of anhydrous KF (potassium fluoride) (purity of 99%, particle size of 20-50 μm) were added. 60 g of solvent was removed by stirring distillation under reduced pressure (130-135 °C/60 mmHg), and the system was heated to 90 °C after the moisture content was determined to be less than 1000 ppm by water evaporation. Subsequently, 129.40 g of pentachloropyridine of 97% purity was added, the reaction temperature was maintained at 90 °C and stirred for 1.5 hours. Upon completion of the reaction, the heating was stopped and the reaction mixture was cooled to room temperature. The reaction mixture was filtered and the filtrate and solid residue were obtained by separation. The solid residue was washed with 100 mL of DMI and diafiltrated to obtain the wash solution. The wash solution and initial filtrate were combined and a distillation operation was carried out to collect the fractions at a pressure of 65-70.5 KPa to obtain the target product 3,5-dichloro-2,4,6-trifluoropyridine.

2176-62-7
268 suppliers
$12.00/10g

1737-93-5
261 suppliers
$30.80/5g
Yield:1737-93-5 95.42%
Reaction Conditions:
with potassium fluoride at 90; for 1.5 h;Temperature;Reagent/catalyst;Concentration;
Steps:
12 Example 12: A process for the preparation of 3,5-dichloro-2,4,6-trifluoropyridine,Specific operations are as follows:
In a 1000 mL three-necked flask, add 600 mL of DMI and anhydrous KF146.72 g with a purity of 99% and a particle size of 20-50 um. Stirring and distilling under reduced pressure (130-135 ° C / 60 mmHg)60 g solvent with water evaporation, the measured water content of less than 1000 ppm, heated to 90 ° C, adding purity of 97% of the five chloropyridine 129.40 g, After 1.5 h of incubation, heating was stopped, the reaction mixture was cooled and the reaction mixture was filtered to obtain filtrate and residue. The residue was washed with 100 mL of DMI and suction filtered to obtain a washing solution. Combined washing liquid and filtrate, distillation, collecting 65 ~ 70 , 5 KPa fractions, namely 3,5-dichloro-2,4,6-trifluoropyridine
References:
Sichuan Fusida Biotechnology Development Co., Ltd;Luo, Qian;Peng, Zhou;Li, Zhou;Wang, Lei;Zhang, Hua;Pi, Yawei CN106008331, 2016, A Location in patent:Paragraph 0059; 0063

2176-62-7
268 suppliers
$12.00/10g
5958-25-8
0 suppliers
inquiry
17717-16-7
4 suppliers
inquiry

1737-93-5
261 suppliers
$30.80/5g

17717-16-7
4 suppliers
inquiry

1737-93-5
261 suppliers
$30.80/5g

2176-62-7
268 suppliers
$12.00/10g
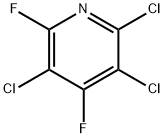
34415-31-1
29 suppliers
$499.19/5MG

1737-93-5
261 suppliers
$30.80/5g

2176-62-7
268 suppliers
$12.00/10g

34415-31-1
29 suppliers
$499.19/5MG

1737-93-5
261 suppliers
$30.80/5g
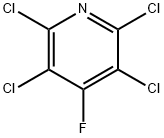
34415-32-2
14 suppliers
$690.79/1g