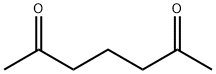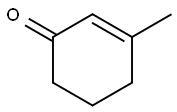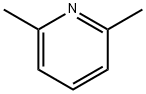
2,6-Lutidine synthesis
- Product Name:2,6-Lutidine
- CAS Number:108-48-5
- Molecular formula:C7H9N
- Molecular Weight:107.15
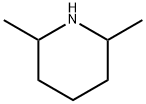
504-03-0
177 suppliers
$5.00/25mg

108-48-5
489 suppliers
$9.90/190μL
Yield:108-48-5 >99 %Chromat.
Reaction Conditions:
in octane at 120; under 760.051 Torr; for 18 h;Inert atmosphere;Schlenk technique;
Steps:
Typical procedure for the acceptorless dehydrogenation of cyclic amines:
General procedure: Into a Schlenk tube (volume: ca. 20 mL) connected to a balloon partially filled with Ar gas, Pd/LDH (75 mg, Pd: 3 mol %), 4-isopropylpiperidine (0.5 mmol), decane (internal standard, 0.1 mmol), octane (2.0 mL), and a Teflon-coated magnetic stir bar were successively placed, and the reaction mixture was vigorously stirred at 120 °C, in 1 atm of Ar. After the reaction was completed, the conversion of 4-isopropylpiperidine and the yield of 4-isopropylpyridine were determined by GC analysis. The reactions of piperidine and 4-methylpiperidine were conducted in a test tube with an Ar balloon. During these reactions, the upper side of the test tube was cooled by refrigerant (-5 °C).
References:
Oyama, Takashi;Yatabe, Takafumi;Jin, Xiongjie;Mizuno, Noritaka;Yamaguchi, Kazuya [Chemistry Letters,2019,vol. 48,# 6,p. 517 - 520] Location in patent:supporting information
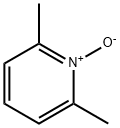
1073-23-0
178 suppliers
$14.00/1g

108-48-5
489 suppliers
$9.90/190μL
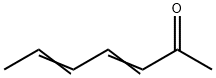
3916-64-1
40 suppliers
$65.00/100mg

108-48-5
489 suppliers
$9.90/190μL
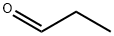
123-38-6
455 suppliers
$14.00/5mL
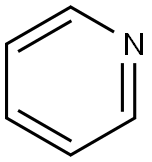
110-86-1
888 suppliers
$9.00/5g

108-48-5
489 suppliers
$9.90/190μL
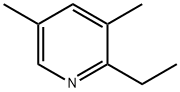
1123-96-2
32 suppliers
inquiry
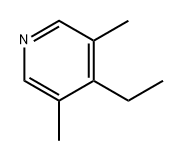
2687-88-9
0 suppliers
inquiry