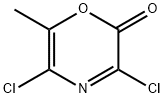
3,5-DICHLORO-6-METHYL-1,4-OXAZIN-2-ONE synthesis
- Product Name:3,5-DICHLORO-6-METHYL-1,4-OXAZIN-2-ONE
- CAS Number:125849-94-7
- Molecular formula:C5H3Cl2NO2
- Molecular Weight:179.99

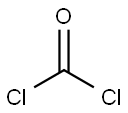
79-37-8
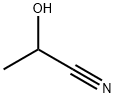
78-97-7

125849-94-7
A solution of oxalyl chloride (179 g, 1.41 mol) in toluene (303 mL) was added to a three-necked round-bottomed flask under nitrogen protection and cooled to 0 °C. Subsequently, a toluene (118 mL) solution of DL-lactonitrile (25.0 g, 352 mmol) was added dropwise for a controlled time of 20 minutes. The reaction mixture was stirred continuously at 0 °C for 50 min before being transferred to a 70 °C oil bath and slowly warmed to about 95 °C. At this temperature, triethylamine hydrochloride (16.8 g, 176 mmol) was added cautiously, taking care to control the rate of addition to prevent violent exotherm. After addition, the reaction system was stirred at 100 °C for 18 hours. Upon completion of the reaction, the solvent was removed by rotary evaporator in a 60 °C water bath. The crude product was extracted with diethyl ether (about 2 L) to isolate a solution containing the target product 3,5-dichloro-6-methyl-1,4-oxazol-2-one and impurities. The solid residue was again extracted with diethyl ether (2 L) to obtain a solution of the target product with higher purity. The solutions from the two extractions were concentrated to dryness by rotary evaporation, respectively. The product containing impurities was cooled to 0 °C and precipitated as a yellow solid, which was removed as a dark red oil by decantation. This yellow solid was combined with the yellow solid from the second extraction to afford the target product 3,5-dichloro-6-methyl-1,4-oxazol-2-one (45.5 g, 253 mmol, 72% yield). The structure of the product was confirmed by 1H NMR (400 MHz, DMSO-d6), δppm 2.30 (s, 3H).

78-97-7
0 suppliers
$66.09/25ml

125849-94-7
28 suppliers
$130.00/100mg
Yield:-
Reaction Conditions:
with oxalyl dichloride;triethylamine hydrochloride in chlorobenzene at 0 - 90;Inert atmosphere;
Steps:
1 Step 1: 3,5-Dichloro-6-methyl-2H-1,4-oxazin-2-one
Into a 10-L 4-necked round-bottom flask was charged oxalic dichloride (3.32 kg, 26.2 mol) and chlorobenzene (3.5 L) under an inert atmosphere of nitrogen. A solution of 2- hydroxypropanenitrile (464.8 g, 6.54 mol) in chlorobenzene (500 mL) was added dropwise to the flask at 0 °C. The system was heated to 90 °C and triethylamine hydrochloride (66.2 g, 481 mmol) was added in portions at 90 °C. The resulting solution was stirred for 3 h before concentrating the mixture under reduced pressure. The resulting solution was diluted with ether (5 L) and the solids were filtered out. The filtrate concentrated and was then applied purified by silica gel column chromatography (0:1-1:4 EtOAc:petroleum ether) to yield the title compound.
References:
MERCK SHARP & DOHME CORP.;GAO, Xiaolei;KNOWLES, Sandra, L.;LI, Chunsing;LO, Michael Man-Chu;MAZZOLA, Robert, D., Jr.;ONDEYKA, Debra, L. WO2018/118736, 2018, A1 Location in patent:Page/Page column 53; 54

79-37-8
490 suppliers
$17.67/10gm:

78-97-7
0 suppliers
$66.09/25ml

125849-94-7
28 suppliers
$130.00/100mg