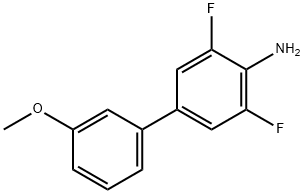
3,5-DIFLUORO-3-METHOXY-[1,1-BIPHENYL]-4-AMINE synthesis
- Product Name:3,5-DIFLUORO-3-METHOXY-[1,1-BIPHENYL]-4-AMINE
- CAS Number:867288-00-4
- Molecular formula:C13H11F2NO
- Molecular Weight:235.23
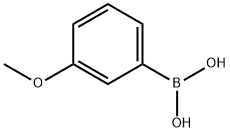
10365-98-7
418 suppliers
$9.00/10g
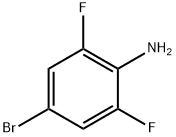
67567-26-4
349 suppliers
$5.00/1g
![3,5-DIFLUORO-3-METHOXY-[1,1-BIPHENYL]-4-AMINE](/CAS/20180527/GIF/867288-00-4.gif)
867288-00-4
16 suppliers
inquiry
Yield:867288-00-4 61%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium carbonate in 1,4-dioxane;water; for 3.5 h;Inert atmosphere;
Steps:
13 Example 13, Preparation of 2.6-Difluoro-(3-methoxy-[1,1'-biphenyl]-4-amino (Intermediate 1m)
4-bromo-2.6-difluoro-aniline 1 g (4.81 mmol) and m-methoxyphenylboronic acid 730 mg (4.81 mmol. 1 eq)Potassium carbonate (4.82 g 24.88 mmol 6 eq) [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (425.35 mg481 mmol 6 eq) was placed in a 100 ml two-necked flask, and 30 ml (dioxane: water = 3:1) was injected with nitrogen to protect the solution, and 80 was allowed to react for 3.5 h. After the TLC monitoring reaction is completed, the post-treatment distillation is carried out under reduced pressure to about 10 ml, and the solution is poured into a separating funnel, extracted with dichloromethane for 2-3 times, dried over anhydrous Na 2 SO 4 , filtered, and evaporated to dryness to give a black oily drop. Separated and purified by petroleum ether: ethyl acetate = 6:1 silica gel column chromatography to obtain a pale yellow oily liquid about 690 mg, yield 61%.
References:
CN108467370,2018,A Location in patent:Paragraph 0077; 0078; 0079