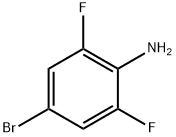
4-Bromo-2,6-difluoroaniline synthesis
- Product Name:4-Bromo-2,6-difluoroaniline
- CAS Number:67567-26-4
- Molecular formula:C6H4BrF2N
- Molecular Weight:208
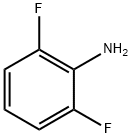
5509-65-9

67567-26-4
b) Preparation of Intermediate 2: Dissolve 2,6-difluoroaniline (3.0 g, 22.56 mmol) in acetic acid (10 mL). Bromine (1.2 mL) was slowly added dropwise to the above solution. The reaction mixture was stirred at room temperature for 15 minutes. After the reaction was completed, the solvent was evaporated under reduced pressure. The residue was neutralized with aqueous sodium carbonate and the aqueous phase was subsequently extracted with dichloromethane. The organic phases were combined, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give Intermediate 2 in 92% yield.

5509-65-9
494 suppliers
$10.00/10g

67567-26-4
350 suppliers
$5.00/1g
Yield:67567-26-4 92%
Reaction Conditions:
Stage #1:2,6-difluoroaniline with bromine;acetic acid in water at 20; for 0.25 h;
Stage #2: with sodium carbonate in water
Steps:
A1.b
b) Preparation of intermediate 2; 2,6-difluorobenzeneamine (3.0g, 22.56 mmoles) was dissolved in acetic acid (10 ml). Bromine (1.2 ml) was added to the solution. The mixture was stirred for 15 minutes at room temperature. After evaporation of the solvent, the residue was treated with an aqueous solution of sodium carbonate. The aqueous solution was extracted with dichloromethane. The organic extract was dried over MgSC>4 and was evaporated. Yield : 92% of intermediate 2.
References:
JANSSEN PHARMACEUTICA N.V.;ARTS, Frank, Xavier, Jozef, Herwig WO2006/15985, 2006, A1 Location in patent:Page/Page column 54
289-95-2
330 suppliers
$15.00/1g

5509-65-9
494 suppliers
$10.00/10g

67567-26-4
350 suppliers
$5.00/1g