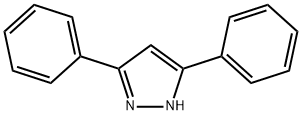
3,5-DIPHENYLPYRAZOLE synthesis
- Product Name:3,5-DIPHENYLPYRAZOLE
- CAS Number:1145-01-3
- Molecular formula:C15H12N2
- Molecular Weight:220.27
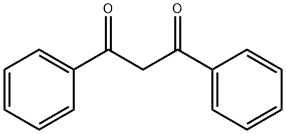
120-46-7
427 suppliers
$6.00/25g

1145-01-3
117 suppliers
$10.00/1g
Yield:1145-01-3 98%
Reaction Conditions:
with carbazic acid at 90; for 5 h;Green chemistry;
Steps:
3. General procedure for the solid state or solvent-free reactions between 1a and di-carbonyl compounds.
General procedure: A 10.0 mmol for α-keto acid compound or a 5.2 mmol for β-diketones or α-keto acids was mixed with 0.40 g of hydrazinium carboxylate (1a, 5.2 mmol), respectively. (For solid di-carbonyl compound, the mixture was ground using a pestle and a mortar.) The mixture was stored in a closed vial, and then heated to 70 - 90 °C until the reaction was complete. Complete conversion to related product was dependent upon the nature of di-carbonyl compounds. Typically, those di-carbonyl compounds take about <3 h to complete the reactions. CO2 and water were released during the reaction. All products obtained from the reactions of 1a with di-carbonyl compounds were basically characterized by 1H and 13C NMR spectroscopy. The products have over 97% of purity of reaction mixture based on 1H NMR spectroscopy and isolation yields are over 97% based on di-carbonyl compounds. The melting points, elemental analysis and UV-Vis spectra for all azines, pyrazoles and pyridazinones, were measured after purification using appropriate solvent.
References:
Lee, Byeongno;Kang, Philjun;Lee, Kyu Hyung;Cho, Jaeheung;Nam, Wonwoo;Lee, Won Koo;Hur, Nam Hwi [Tetrahedron Letters,2013,vol. 54,# 11,p. 1384 - 1388] Location in patent:supporting information
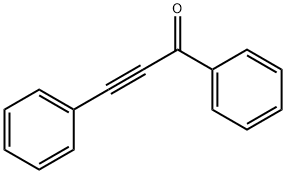
7338-94-5
42 suppliers
$220.00/100mg

1145-01-3
117 suppliers
$10.00/1g
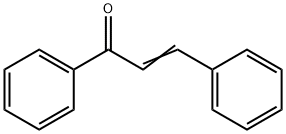
94-41-7
209 suppliers
$10.00/5g

1145-01-3
117 suppliers
$10.00/1g
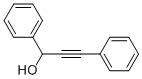
1817-49-8
31 suppliers
$29.09/1g

1145-01-3
117 suppliers
$10.00/1g
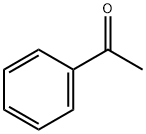
98-86-2
795 suppliers
$9.00/5g

1145-01-3
117 suppliers
$10.00/1g