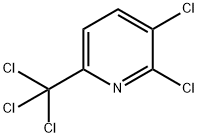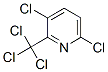
3,6-DICHLORO-2-(TRICHLOROMETHYL)PYRIDINE synthesis
- Product Name:3,6-DICHLORO-2-(TRICHLOROMETHYL)PYRIDINE
- CAS Number:1817-13-6
- Molecular formula:C6H2Cl5N
- Molecular Weight:265.35
Yield:-
Reaction Conditions:
with chlorine;Cs-ion exchanged Type L zeolite at 200; under 25.8581 Torr; for 8 h;
Steps:
1 CATALYST EVALUATION TEST METHOD EXAMPLE 1 Standard Operating Conditions
Nitrogen was flow-controlled at a rate of 10.00 standard cubic centimeters per second (cm3/min) over the headspace of tube-shaped glass evaporators containing α-6-tet. The evaporator temperature was controlled at 100° C. with a GC convection oven to give desired α-6-tet vapor pressure of 0.003 atmospheres (atm). The α-6-tet and nitrogen sweep gas then mixed with chlorine that was flow controlled at 5.00 standard cm3/min, and then fed to a GC oven containing rod-shaped glass reactor tubes of 0.25 inch O.D. partially filled with catalyst. The catalyst beds ranged from 0.10-0.60 g and 1.5-7.0 centimeters (cm) in length. The reactor temperature was controlled at designated temperatures (175-325° C.) with a GC convection oven. The product streams from the reactor were sent to an organic trap/vent system. The organics trap system consisted of a glass alligator flask with overhead tubing that sent residual chlorine to glass scrubber bottles containing 10% caustic. Samples from the product stream were analyzed by gas chromatography. The scrubber system was open to atmopheric pressure. [0038] Before generating catalytic conversion data, all the catalysts were pretreated to remove residual water from the surfaces. The conditioning consisted of feeding the α-6-tet/nitrogen sweep and Cl2 over the freshly loaded catalyst bed at 10.00 and 5.00 standard cm3/min, respectively. The reactor temperature was set at 175° C. The conditioning process was monitored by GC-Mass Spec Analysis and typically lasted from 1-5 hours. Complete conditioning was indicated by diminished acid chloride levels.
References:
US2004/176607,2004,A1 Location in patent:Page 3-4
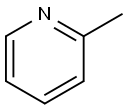
109-06-8
419 suppliers
$16.00/25g
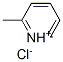
14401-91-3
4 suppliers
inquiry

1817-13-6
4 suppliers
inquiry
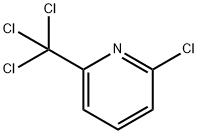
1929-82-4
361 suppliers
$25.00/1g

109-06-8
419 suppliers
$16.00/25g

14401-91-3
4 suppliers
inquiry
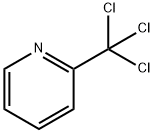
4377-37-1
17 suppliers
inquiry

1817-13-6
4 suppliers
inquiry

1929-82-4
361 suppliers
$25.00/1g

14401-91-3
4 suppliers
inquiry

4377-37-1
17 suppliers
inquiry
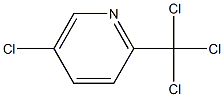
1197-03-1
0 suppliers
inquiry
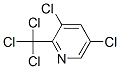
1128-16-1
2 suppliers
inquiry

1817-13-6
4 suppliers
inquiry

1929-82-4
361 suppliers
$25.00/1g
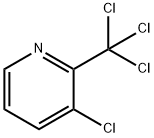
1817-12-5
0 suppliers
inquiry