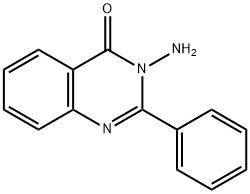
3-AMINO-2-PHENYL-4(3H)-QUINAZOLINONE synthesis
- Product Name:3-AMINO-2-PHENYL-4(3H)-QUINAZOLINONE
- CAS Number:1904-60-5
- Molecular formula:C14H11N3O
- Molecular Weight:237.26
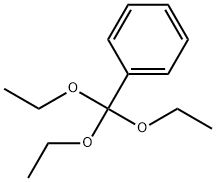
1663-61-2
186 suppliers
$10.00/5g
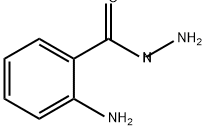
1904-58-1
106 suppliers
$45.00/10g

1904-60-5
15 suppliers
$15.00/5mg
Yield:1904-60-5 95%
Reaction Conditions:
with acetic acid in ethanol at 110; for 24 h;Inert atmosphere;Sealed tube;
Steps:
3.2.2. Method 2
General procedure: The 2-aminobenzamide (1 equiv), the orthoester (2-3 equiv) and absolute ethanol (2-3 mL) wereplaced in a 15-mL Chemglass screw-cap pressure tube (No. CG-1880-01, Chemglass, Vineland, NJ,USA). Glacial acetic acid (3 equiv) was added, N2 was introduced to the vessel and the cap wastightened. The vessel was heated at 110 °C for 12-72 h, then cooled and concentrated to give thequinazolinone, which was purified by crystallization from absolute ethanol or trituration from 5%ether in pentane. The following compounds were prepared:
References:
Gavin, Joshua T.;Annor-Gyamfi, Joel K.;Bunce, Richard A. [Molecules,2018,vol. 23,# 11,art. no. 2925]
3084-52-4
3 suppliers
inquiry

1904-60-5
15 suppliers
$15.00/5mg
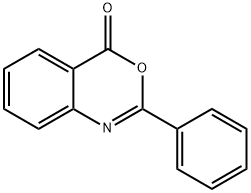
1022-46-4
70 suppliers
$90.00/25mg

1904-60-5
15 suppliers
$15.00/5mg
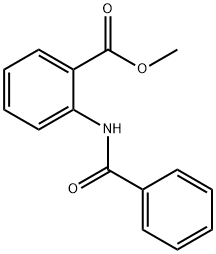
7510-49-8
20 suppliers
$28.60/250mg

1904-60-5
15 suppliers
$15.00/5mg
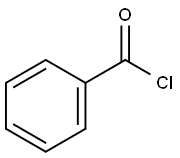
98-88-4
561 suppliers
$10.00/5g

1904-60-5
15 suppliers
$15.00/5mg