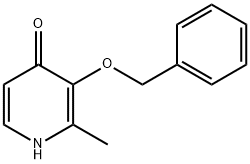
3-(BENZYLOXY)-2-METHYL-4(1H)-PYRIDINONE synthesis
- Product Name:3-(BENZYLOXY)-2-METHYL-4(1H)-PYRIDINONE
- CAS Number:61160-18-7
- Molecular formula:C13H13NO2
- Molecular Weight:215.25
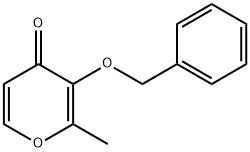
61049-69-2

61160-18-7
General procedure for the synthesis of 3-(benzyloxy)-2-methyl-4H-pyran-4-one from 3-(benzyloxy)-2-methyl-4H-pyran-4-one: To a solution of 3-(benzyloxy)-2-methyl-4H-pyran-4-one (13.8 g, 0.064 mol) in ethanol (25 mL) was added aqueous ammonia solution (50 mL), and the reaction was carried out at reflux overnight. After completion of the reaction, the solvent was removed by distillation under reduced pressure. The residue was dissolved in water and the pH was adjusted with concentrated hydrochloric acid to 1. The resulting aqueous phase was washed with ethyl acetate (3 x 25 mL), followed by adjusting the pH with 2 M sodium hydroxide solution to 10. The aqueous phase was then extracted with chloroform (3 x 1 L), and the organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was recrystallized with a solvent mixture of methanol/ether to give brown cubic crystals with a melting point of 162-164 °C. The product was extracted with chloroform (3×L) and dried with anhydrous sodium sulfate. The yield was 75%. The product was characterized by 1H NMR (CDCl3): δ 2.15 (3H, s, CH3), 5.03 (2H, s, CH2Ph), 6.35 (1H, d, J = 6.9Hz, 5-H), 7.25-7.31 (5H, m, Ph-H), 7.39 (1H, d, J = 6.9Hz, 6-H). The molecular formula is C13H13NO2.

61160-18-7
76 suppliers
$28.00/250mg
105-13-5
566 suppliers
$5.00/5G
![Pyridine, 4-[(4-methoxyphenyl)methoxy]-2-methyl-3-(phenylmethoxy)-](/CAS/20210305/GIF/865300-87-4.gif)
865300-87-4
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with triphenylphosphine;diethylazodicarboxylate in tetrahydrofuran at 20;
Steps:
1.1
EXAMPLE 1; 2- (4-Fluorobenzyl)-9-hydroxy-3, 4-dihydro-2H-pyrido [1, 2-a] pyrazine-1, 8-dione; Step 1: 3- (Benzyloxy)-4- [ (4-methoxybenzyl) oxy] -2-methylpyridine (A1); DEAD (1.5 equivalent) was added dropwise over 10 minutes to a stirred solution of 3- (benzyloxy) -2-methylpyridin-4-ol (1.0 equivalent), 4-methoxybenzylalcohol (1.3 equivalents) and triphenylphosphine (1.5 equivalents) in THF at room temperature. The mixture was stirred overnight and then the solvent was removed under reduced pressure. The resulting mixture was triturated with EtOAc and hexanes, and filtered. The solution was concentrated under reduced pressure and then purified by column chromatography on silica eluting with 100% EtOAc to yield the desired pyridine Al. 1H NMR (400 MHz, CDC13) 5 8.15 (1H, d, J = 5.6 Hz), 7.72-7. 30 (7H, m), 6.96 (2H, d, J = 7.8 Hz), 6.83 (1H, d, J = 5.6 Hz), 5.15 (2H, s), 4.98 (2H, s), 3.74 (3H, s), 2.45 (3H, s). MS (ES) C2lH2lNO3requires : 335, found: 336 (M+H+).
References:
WO2005/87766,2005,A1 Location in patent:Page/Page column 52-53