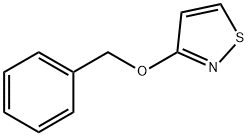
3-(Benzyloxy)isothiazole synthesis
- Product Name:3-(Benzyloxy)isothiazole
- CAS Number:60666-83-3
- Molecular formula:C10H9NOS
- Molecular Weight:191.25

100-39-0
424 suppliers
$10.00/10g
1003-07-2
109 suppliers
$35.00/100mg

60666-83-3
18 suppliers
inquiry
Yield:60666-83-3 27%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 0 - 25; for 24 h;
Steps:
44.3 Step 3: 53-D
To a solution of 53-C (7.3 g, 72.19 mmol) in DMF (50 mL) at 0 C was added K2CO3(19.95 g, 144.37 mmol) followed by benzyl bromide (14.2 mL, 83.01 mmol) . The reaction mixture was allowed to stir for 24 hr at 25 C. The reaction mixture was diluted with H2O (200 mL) and extracted with Et2O. The organic layer was washed with brine, dried over sodium sulfate, and evaporated to give a crude product, which was purified by column flash chromatography (heptane/EA = 4/1) to give 53-D (3.78 g, 27%yield) .1H NMR (400 MHz, CDCl3) δ 8.48 (d, J = 4.7 Hz, 1H) , 7.49 (dd, J = 7.7, 1.0 Hz, 2H) , 7.45 -7.32 (m, 3H) , 6.67 (d, J = 4.7 Hz, 1H) , 5.45 (s, 2H) ; MS: m/z = 191.9 (M + 1) .
References:
WO2022/17338,2022,A1 Location in patent:Paragraph 00564

100-39-0
424 suppliers
$10.00/10g

1003-07-2
109 suppliers
$35.00/100mg

60666-83-3
18 suppliers
inquiry