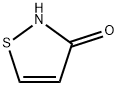
thiazol-3-one synthesis
- Product Name:thiazol-3-one
- CAS Number:1003-07-2
- Molecular formula:C3H3NOS
- Molecular Weight:101.13
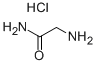
1668-10-6

1003-07-2
General procedure for the synthesis of isothiazol-3-one from glycinamide hydrochloride: glycinamide hydrochloride (1 mol) was suspended in N,N-dimethylformamide (DMF, 500 mL), and dichlorosulfoxide (SO2Cl2, 300 mL) was slowly added dropwise over a period of 1.5 h, while cooling to maintain the reaction temperature at 5-10 °C. The reaction was carried out with stirring. After the dropwise addition, the reaction was continued with stirring at 10-15 °C. Subsequently, water (500 mL) was added slowly over a period of 6 hours. After completion of the reaction, the solid insoluble material was removed by filtration and the filtrate was extracted with ether (2 L). The ether extracts were combined and washed with saturated saline (200 mL), followed by evaporation of the solvent under reduced pressure to give a light yellow solid (132 g, Product A). The aqueous layer was extracted with dichloromethane (DCM, 2 x 600 mL), and the DCM extracts were combined and washed with ether and water sequentially. The organic layer was then washed with saturated saline and the solvent was evaporated under reduced pressure to give a milky white solid (18 g, Product B). Products A and B were combined, dissolved in ether, dried by adding desiccant and decolorized by adding activated carbon. The activated carbon was removed by filtration and the filtrate was evaporated under reduced pressure to give a light yellow solid (104.3 g). The solid was ground with isohexane to give the final isothiazol-3-one (91.3 g, 90% yield). Melting point: 102-105 °C.

1668-10-6
509 suppliers
$5.00/5g

1003-07-2
119 suppliers
$35.00/100mg
Yield: 90%
Reaction Conditions:
with sulfuryl dichloride in DMF (N,N-dimethyl-formamide) at 5 - 15; for 7.5 h;
Steps:
76 Method 76; 3-Oxo-2,3-dihydroisothiazole
Glycinamide.HCl (1 mol) was suspended in DMF (500 ml) and SO2Cl2 (300 ml) was added dropwise over 1.5 hours with cooling keeping the reaction temperature between 5-10° C. The reaction was stirred at 10-15° C. for 6 hours when water (500 ml) was added cautiously. The solid was removed by filtration and the filtrate extracted with ether (21). The Ethereal solution was washed brine (200 ml) and evaporated in vacuo to yield a pale yellow solid (132 g)-A. The aqueous layer was extracted with DCM (2*600 ml). The DCM portions were combined and washed with ether and water. The organic layer was washed brine and evaporated in vacuo to yield a cream solid (18 g)-B. A & BD were combined, dissolved in ether, dried and charcoal was added. The solution was filtered and the filtrate evaporated in vacuo to yield a pale yellow solid (104.3 g). This solid was triturated with isohexane to yield the title compound (91.3 g, 90%). Mpt: 102-5° C.
References:
Breault, Gloria Anne;Newcombe, Nicholas John;Thomas, Andrew Peter US2004/14776, 2004, A1 Location in patent:Page/Page column 37-38
![3-[(3-AMINO-3-OXOPROPYL)DITHIO]PROPANAMIDE](/CAS/GIF/1002-19-3.gif)
1002-19-3
50 suppliers
$75.00/100mg
25629-58-7
67 suppliers
$45.00/100mg

1003-07-2
119 suppliers
$35.00/100mg
2807-36-5
0 suppliers
inquiry
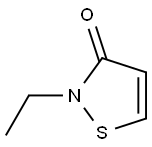
2682-21-5
0 suppliers
inquiry
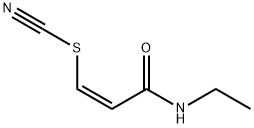
2682-36-2
0 suppliers
inquiry

1003-07-2
119 suppliers
$35.00/100mg