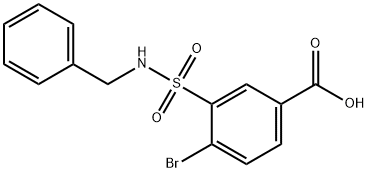
3-(benzylsulfamoyl)-4-bromobenzoic acid synthesis
- Product Name:3-(benzylsulfamoyl)-4-bromobenzoic acid
- CAS Number:312758-94-4
- Molecular formula:C14H12BrNO4S
- Molecular Weight:370.22
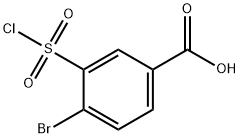
50803-23-1
37 suppliers
$45.00/100mg

100-46-9
501 suppliers
$5.00/5G

312758-94-4
1 suppliers
inquiry
Yield:312758-94-4 23%
Reaction Conditions:
in tetrahydrofuran; for 18 h;Reflux;
Steps:
2 3-Benzylsulfamoyl-4-bromo-benzoic acid (JK-4-36)
3-Benzylsulfamoyl-4-bromo-benzoic acid (JK-4-36): 4-bromobenzoic acid (0140) (1.7 g, 8.5 mmol) was added drop-wise to chlorosulfonic acid (4.3 mL, 8.5 mmol) at 0°C. After addition was finished reaction mixture was heated to 130°C for 10 h. Cooled reaction mixture was added drop-wise to 85 mL of ice water. Formed precipitate was filtered off and washed with cold water. Solid was dissolved in diethyl ether and dried with sodium sulfate. Solvent was evapotarted giving 4-bromo-3-chlorosulfonyl-benzoic acid (2 g, 6.67 mmol, 79%>) as beige solid. 4-bromo-3-chlorosulfonyl-benzoic acid was dissolved in 8 mL of THF. To this solution benzylamine (0.73 mL, 6.67 mmol) was added drop-wise and reaction was refluxed for 18 h. After that time 15 mL of ethyl acetate and 15 mL of 1 M NaOH were added. Organic phase was further extracted with two 20 mL portions of 1 M NaOH. Aqueous phases were combined, pH adjusted to approx. 2 with 1 M HC1 and extracted with three 15 mL portions of ethyl acetate. Organic phases were dried with sodium sulfate. Evaporation of solvent gave 568mg (23%) of 3-benzylsulfamoyl-4-bromo-benzoic acid as white solid. 1H- NMR (400 MHz, DMSO- 6): δ 8.55 (t, J = 6.1 Hz, 1H), 8.40 (d, J = 1.9 Hz, 1H), 7.92 (m, 2H), 7.16 (m, 5H), 4.14 (d, J = 4.0 Hz, 2H). 13C-NMR (100 MHz, DMSO-d6): δ 166.4, 140.7, 140.6, 137.7, 136.0, 134.2, 131.4, 128.5, 128.0, 127.5, 124.0, 46.6.
References:
WO2015/138377,2015,A1 Location in patent:Paragraph 00113
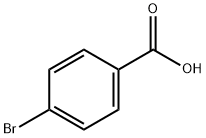
586-76-5
494 suppliers
$10.00/1g

312758-94-4
1 suppliers
inquiry