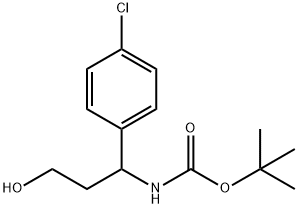
3-(Boc-aMino)-3-(4-chlorophenyl)-1-propanol synthesis
- Product Name:3-(Boc-aMino)-3-(4-chlorophenyl)-1-propanol
- CAS Number:886493-66-9
- Molecular formula:C14H20ClNO3
- Molecular Weight:285.77

24424-99-5
869 suppliers
$13.50/25G
68208-26-4
63 suppliers
$60.00/100mg

886493-66-9
14 suppliers
inquiry
Yield:886493-66-9 99%
Reaction Conditions:
in dichloromethane at 22; for 2 h;
Steps:
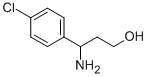
Intermediate 30: tert-butyl l-(4-chlorophenyl)-3-hvdroxypropylcarbamateDi-tert-butyl dicarbonate (0.705 g, 3.23 mmol) was added to 3-amino-3-(4- chlorophenyl)propan-l-ol (Intermediate 29) (0.500 g, 2.69 mmol) in DCM (30 mL) at 220C. The resulting solution was stirred at 22 0C for 2 hours. The mixture was concentrated and the residue was purified by flash silica chromatography, elution gradient 0 to 4% (10:1 MeOH/conc. NH3 (aq)) in DCM. Pure fractions were evaporated to dryness to afford tert-butyl l-(4-chlorophenyl)-3-hydroxypropylcarbamate (0.759 g, 99 %) as a white solid.IH NMR (399.902 MHz, CDC13) δ 1.43 (9H, s), 1.81 (IH, m), 2.04 (IH, m), 2.74 (IH, br.s), 3.69 (2H, m), 4.88 (IH, br.s), 5.04 (IH, d), 7.23 (2H, d), 7.32 (2H, d). MS m/e MH" 284, 286
References:
WO2009/47563,2009,A1 Location in patent:Page/Page column 103
19947-39-8
164 suppliers
$15.00/1g

886493-66-9
14 suppliers
inquiry