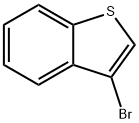
3-Bromo-1-benzothiophene synthesis
- Product Name:3-Bromo-1-benzothiophene
- CAS Number:7342-82-7
- Molecular formula:C8H5BrS
- Molecular Weight:213.09
![2,3-Dibromobenzo[b]thiophene](/CAS/GIF/6287-82-7.gif)
6287-82-7

7342-82-7
The general procedure for the synthesis of 3-bromobenzothiophene from 2,3-dibromothiophene was as follows: first, the halogenated heterocycle (0.66 mmol) was dissolved in anhydrous THF (13.2 mL) and degassed by drumming in argon for a few minutes. Subsequently, PdCl2 (dppf) (27.0 mg, 0.033 mmol, 5.0 mol%), TMEDA (0.130 g, 1.12 mmol, 1.7 equiv) and NaBH4 (42.4 mg, 1.12 mmol, 1.7 equiv) were added sequentially. The reaction mixture was stirred at room temperature for appropriate time under argon protection and finally post-treated according to standard methods.
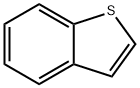
95-15-8
395 suppliers
$6.00/5g

7342-82-7
190 suppliers
$5.00/1g
Yield:7342-82-7 100%
Reaction Conditions:
with N-Bromosuccinimide;acetic acid in chloroform at 0 - 20; for 52 h;
Steps:
18.1 Step 1: 3-Bromo-1-benzothiophene
To a solution of benzo[b]thiophene (10 g, 74.5 16 mmol) in chloroform (75 mL) and acetic acid (75 mL), was stepwise added NBS (16.6 g, 93.268 mmol) for 4 hr at 0°C and the mixture allowed to stir at room temperature for 48 hr. The progress of the reaction was monitored by TLC and, after completion of reaction, the reaction mass was diluted with chloroform (200 mL) and the resulting mixture was successively washed with a saturated solution of sodium thiosulfate (200 mL), sodium carbonate (200 mL) and brine (150 mL). The extracted organic layer was then dried over sodium sulfate, filtered and evaporated under reduced pressure. The resulting red liquid was then filtered of a pad of silica gel, eluting with hexane to afford 3-bromo-1-benzothiophene (15.87 g, 74.474 mmol, 100%) as yellow oil. The ‘H NMR and mass was confirmed by reported literature.
References:
CURADEV PHARMA PRIVATE LTD.;BANERJEE, Monali;MIDDYA, Sandip;SHRIVASTAVA, Ritesh;RAINA, Sushil;SURYA, Arjun;YADAV, Dharmendra B.;YADAV, Veejendra K.;KAPOOR, Kamal Kishore;VENKATESAN, Aranapakam;SMITH, Roger A.;THOMPSON, Scott K. WO2014/186035, 2014, A1 Location in patent:Page/Page column 186; 187
![2,3-Dibromobenzo[b]thiophene](/CAS/GIF/6287-82-7.gif)
6287-82-7
96 suppliers
$9.00/250mg

7342-82-7
190 suppliers
$5.00/1g
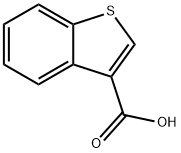
5381-25-9
184 suppliers
$8.00/250mg

7342-82-7
190 suppliers
$5.00/1g
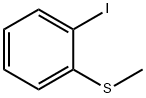
33775-94-9
103 suppliers
$16.00/250mg

7342-82-7
190 suppliers
$5.00/1g
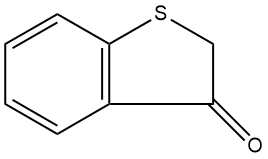
130-03-0
72 suppliers
$28.00/100mg

7342-82-7
190 suppliers
$5.00/1g