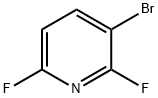
3-Bromo-2,6-difluoropyridine synthesis
- Product Name:3-Bromo-2,6-difluoropyridine
- CAS Number:80392-79-6
- Molecular formula:C5H2BrF2N
- Molecular Weight:193.98
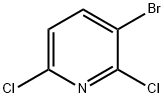
866755-20-6

80392-79-6
GENERAL METHOD: To a solution of 2,6-dichloro-3-bromopyridine (4.70 g, 20.7 mmol) in dimethyl sulfoxide (DMSO, 103 mL) was added cesium fluoride (12.6 g, 82.9 mmol) at room temperature. The reaction mixture was stirred in air at 80 °C for 8 hours. After completion of the reaction, the mixture was poured into room temperature water and extracted with ether (Et2O). The organic layer was separated, washed sequentially with water and saturated saline, dried over anhydrous sodium sulfate (Na2SO4) and subsequently concentrated under reduced pressure (400 Torr, 40°C). Purification of the residue by column chromatography (silica gel, hexane solution of ethyl acetate as eluent) afforded 3-bromo-2,6-difluoropyridine (2.58 g, 64% yield) as a colorless oil.1H NMR (CDCl3) δ: 6.79 (1H, dd, J = 8.3, 3.0 Hz), 8.03 (1H, ddd, J = 8.4, 8.4, 7.0 Hz).19F NMR (CDCl3) δ: -69.3 Hz, -63.8 Hz.Compounds 4B-8B were prepared according to the method similar to 3B.

866755-20-6
149 suppliers
$15.00/1g

80392-79-6
87 suppliers
$11.00/100mg
Yield: 64%
Reaction Conditions:
with cesium fluoride in dimethyl sulfoxide at 80; for 8 h;
Steps:
3-Bromo-2,6-difluoropyridine(3B)
General procedure: To asolution of 3-bromo-2,6-dichloropyridine (4.70 g, 20.7 mmol) in DMSO (103 ml)was added cesium fluoride (12.6 g, 82.9 mmol) at room temperature. The mixturewas stirred at 80 °C under air for 8 h. The mixture was poured into water atroom temperature and extracted with Et2O. The organic layer wasseparated, washed with water and brine, dried over Na2SO4and concentrated in vacuo. (400 Torr, 40 °C). The residue was purified bycolumn chromatography (silica gel, eluted with EtOAc in hexane) to give 3-bromo-2,6-difluoropyridine (3B) (2.58 g, 64%) as colorless oil. 1H NMR (CDCl3)δ: 6.79 1H,dd, J = 8.3, 3.0 Hz), 8.03 (1H, ddd, J = 8.4, 8.4 7.0 Hz). 19F NMR(CDCl3) δ: -69.3 Hz, -63.8 Hz. The compound 4B-8B were prepared in a manner similarto that described for 3B.
References:
Katoh, Taisuke;Tomata, Yoshihide;Tsukamoto, Tetsuya;Nakada, Yoshihisa [Tetrahedron Letters,2015,vol. 56,# 44,p. 6043 - 6046] Location in patent:supporting information
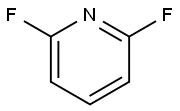
1513-65-1
347 suppliers
$6.00/5g

80392-79-6
87 suppliers
$11.00/100mg
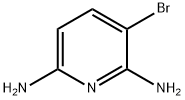
54903-86-5
72 suppliers
$33.00/250mg

80392-79-6
87 suppliers
$11.00/100mg
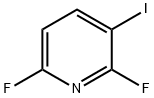
685517-67-3
95 suppliers
$16.00/1g

80392-79-6
87 suppliers
$11.00/100mg
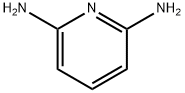
141-86-6
515 suppliers
$6.00/5g

80392-79-6
87 suppliers
$11.00/100mg