
3-BROMO-2-HYDROXY-5-NITRO-6-PICOLINE synthesis
- Product Name:3-BROMO-2-HYDROXY-5-NITRO-6-PICOLINE
- CAS Number:874493-25-1
- Molecular formula:C6H5BrN2O3
- Molecular Weight:233.02
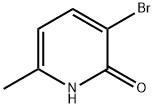
374633-33-7
130 suppliers
$20.00/100mg

874493-25-1
47 suppliers
$18.00/250mg
Yield:874493-25-1 67%
Reaction Conditions:
with nitric acid in water at 20; for 3.5 h;
Steps:
00273j Preparation 13C: 3 -bromo-6-methyl-5 -nitropyridin-2-ol
[00273j Preparation 13C: 3 -bromo-6-methyl-5 -nitropyridin-2-olXNO2[00274j In a 500-mL round-bottomed flask, a 65% aqueous HNO3 was stirred at 0 °C and 3-bromo-6-methylpyridin-2-ol (5.7 g, 30.3 mmol) was introduced dropwise. The reaction mixture was stirred at room temperature for 3.5 h. After pouring the mixture into ice water (200 mL), the aqueous layer was extracted with EtOAc (300 mL x 2). The combined organic layers were washed with water, brine (200 mL x 2), dried over Na2504, filtered, concentrated and purified by column chromatography on silica gel (25%, EtOAc:PE) to give 4.7 g (67%) of the title compound as an off-white solid. ‘HNMR (400 MHz, CDC13) ? 12.85 (s, 3 H), 8.66 (s, 1 H), 2.86 (s, 1 H). [M+H] Calc’d for C6H5BrN2O3, 235, 237; Found, 235, 237.
References:
WO2015/89192,2015,A1 Location in patent:Paragraph 0273; 0274
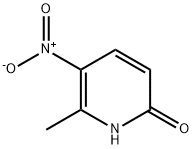
28489-45-4
239 suppliers
$10.00/1g

874493-25-1
47 suppliers
$18.00/250mg
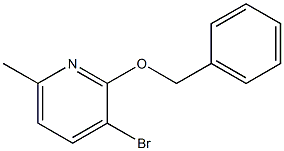
1206774-24-4
6 suppliers
inquiry

874493-25-1
47 suppliers
$18.00/250mg
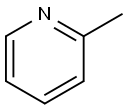
109-06-8
420 suppliers
$16.00/25g

874493-25-1
47 suppliers
$18.00/250mg
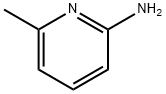
1824-81-3
487 suppliers
$8.00/25g

874493-25-1
47 suppliers
$18.00/250mg