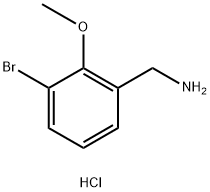
(3-Bromo-2-methoxyphenyl)methanamine hydrochloride synthesis
- Product Name:(3-Bromo-2-methoxyphenyl)methanamine hydrochloride
- CAS Number:1375997-08-2
- Molecular formula:C8H11BrClNO
- Molecular Weight:252.54
![2-[[3-Bromo-2-(methyloxy)phenyl]methyl]-1H-isoindole-1,3(2H)-dione](/CAS/20211123/GIF/1177558-48-3.gif)
1177558-48-3
2 suppliers
inquiry

1375997-08-2
13 suppliers
inquiry
Yield:1375997-08-2 46.3%
Reaction Conditions:
Stage #1: 2-{[3-bromo-2-(methyloxy)phenyl]methyl}-1H-isoindole-1,3(2H)-dionewith hydrazine hydrate in ethanol at 90; for 4 h;
Stage #2: with hydrogenchloride in methanol;water;
Steps:
40
Example 40 {[3-Bromo-2-(methyloxy)phenyl] methyl} amineHydrazine hydrate (7.8 g, 154 mmol) was added to a suspension of 2-{[3-bromo-2- (methyloxy)phenyl]methyl}-lH-isoindole-l,3(2H)-dione (26.7 g, 77.2 mmol) in EtOH (300 mL) and the reaction mixture was heated to 90 0C for 4 h. After cooling to room temperature, the mixture was filtered and the solid was washed with EtOAc (300 mL x 2). The filtrate was evaporated to about 50 mL and filtered again. After removing the solvent, the residue was dissolved in 20 mL of MeOH, and then IN HCl was added to obtain a white solid. Then the white solid was recrystallized from MeOH-Et2O to obtain 9.0 g of the product (yield: 46.3%). 1H NMR (400 MHz, D2O) δ 3.79 (s, 3H), 4.13 (s, 2H), 7.02 (t, J=7.6 Hz, 1 H), 7.27 (d, J=8.0 Hz, 1 H), 7.57 (d, J=8.0 Hz, 1 H); 13C NMR (100 MHz, D2O) δ 37.7, 60.2, 115.6, 125.3, 126.6, 128.8, 133.8, 153.7; MS: m/z 254.1 (M+); HPLC: retention time: 7.618 min; purity: 98.8%.
References:
WO2009/100169,2009,A1 Location in patent:Page/Page column 135
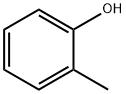
95-48-7
456 suppliers
$19.00/25g

1375997-08-2
13 suppliers
inquiry
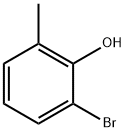
13319-71-6
137 suppliers
$12.00/250mg

1375997-08-2
13 suppliers
inquiry
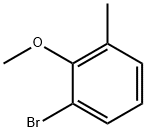
52200-69-8
61 suppliers
$55.00/100mg

1375997-08-2
13 suppliers
inquiry
1177558-47-2
21 suppliers
$135.00/250mg

1375997-08-2
13 suppliers
inquiry