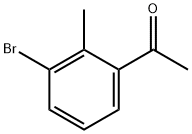
3'-bromo-2'-methylacetophenone synthesis
- Product Name:3'-bromo-2'-methylacetophenone
- CAS Number:52779-76-7
- Molecular formula:C9H9BrO
- Molecular Weight:213.07
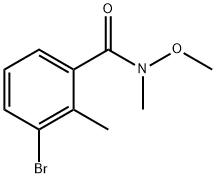
631909-08-5
9 suppliers
inquiry

75-16-1
274 suppliers
$12.00/10ml

52779-76-7
79 suppliers
$32.00/100mg
Yield:52779-76-7 90.9%
Reaction Conditions:
in tetrahydrofuran at 0 - 40; for 3 h;
Steps:
[0245] Step B: To a solution of 33-bromo-N-methoxy-N,2-dimethylbenzamide (120 g, 465 mmol, 1.00 eq.) in THF (100 mL) was added methyl magnesium bromide (3.0 M, 180 mL, 1,16 eq.) at 0 °C. The mixture was stirred between 0-40 °C for 3 hours, then the mixture was cooled to 0 °C and hydrochloric acid (6.0 N) (450 mL) was added dropwise, and stirred for 2 hours between 40-45 °C. Then the mixture was cooled to 25 °C and poured into a saturated ammonium chloride solution (9000 mL). The aqueous phase was extracted with ethyl acetate (1500 mL c 3). The combined organic phase was washed with brine (1000 mL x 3), dried over anhydrous sodium sulfate, filtered, and concentrated under vacuum to give 1-(3-bromo-2-methylphenyl)ethan-1-one (90.0 g, 422 mmol, 90.9% yield) as yellow oil. [0246] NMR (400 MHz, CDiOD) d = 7.70 (dd, J= 1.2, 8.0 Hz, 1H), 7.62 (dd, J= 0.8, 7.6 Hz, 1H), 7.19 (t, J= 8.0 Hz, 1H), 2.56 (s, 3H), 2.46 (s, 3H).
References:
WO2021/127429,2021,A1 Location in patent:Paragraph 0245-0246
6942-39-8
124 suppliers
$34.00/5g

75-16-1
274 suppliers
$12.00/10ml

52779-76-7
79 suppliers
$32.00/100mg

6942-39-8
124 suppliers
$34.00/5g

74-88-4
357 suppliers
$15.00/10g

52779-76-7
79 suppliers
$32.00/100mg
76006-33-2
321 suppliers
$10.00/5g

52779-76-7
79 suppliers
$32.00/100mg