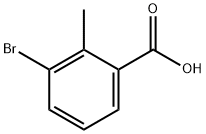
3-Bromo-2-methylbenzoic acid synthesis
- Product Name:3-Bromo-2-methylbenzoic acid
- CAS Number:76006-33-2
- Molecular formula:C8H7BrO2
- Molecular Weight:215.04

124-38-9
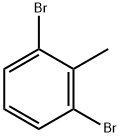
69321-60-4

76006-33-2
Example 27 Synthesis of 3-bromo-2-methylbenzoic acid: tert-butyl lithium (t-BuLi, 1.5 M pentane solution, 17 mL) was slowly added to an anhydrous solution of 1,3-dibromo-2-methylbenzene (6.57 g) in anhydrous tetrahydrofuran (THF, 100 mL) under argon gas protection and the reaction temperature was kept at -80 °C. After the dropwise addition was completed, the reaction mixture was stirred between -76 and -78 °C for 2 hours. Subsequently, the mixture was further cooled below -80 °C, dry ice (solid carbon dioxide) was added and the mixture was allowed to warm up naturally to room temperature. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure, 5% aqueous sodium hydroxide (NaOH) solution (40 mL) was added to the residue, and the aqueous phase was washed with dichloromethane (CH2Cl2, 10 mL x 2). The aqueous phase was acidified with concentrated hydrochloric acid (HCl) to pH=1 and then extracted with ethyl acetate (EtOAc, 100 mL x 2). The organic phases were combined and dried with anhydrous sodium sulfate (Na2SO4). After concentration under reduced pressure, the residue was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate = 8:1 to 1:1) to afford 3.58 g of the target product, 3-bromo-2-methylbenzoic acid, in 63.4% yield. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ2.73 (s, 3H), 7.15 (t, J=8.0 Hz, 1H), 7.77 (dd, J=8.0 Hz, J=1.2 Hz, 1H), 7.94 (dd, J=8.0 Hz, J=1.2 Hz, 1H).
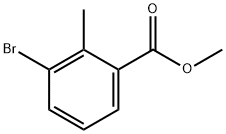
99548-54-6
171 suppliers
$5.00/1g

76006-33-2
321 suppliers
$10.00/5g
Yield:76006-33-2 91%
Reaction Conditions:
with water;lithium hydroxide in tetrahydrofuran at 60; for 16 h;
Steps:
25.1 Step 1 : 3-bromo-2-methylbenzoic acid.
A mixture of methyl 3-bromo-2-methylbenzoate (35.0 g, 152.79 mmol) and LiOH (10.9 g, 453.79 mmol) in THF (300 mL) and H2O (50 mL) was stirred at 60 °C for 16 h and then concentrated under vacuum. The residue was diluted with water (80 mL) and the mixture was then acidified to pH 4 with 2N HC1. The precipitated solids were collected by filtration and washed with water. The solids were dried under vacuum to afford 3-bromo-2-methylbenzoic acid (30 g, 91percent) as a white solid. MS (ESI) calculated for (C8H7Br02) [M+H]+, 214., 9, 216.9; found, 215.0, 217.0. [0676] Step 2: 3-bromophthalic acid.
References:
NURIX THERAPEUTICS, INC.;ROBBINS, Daniel, W.;SANDS, Arthur, T.;MCINTOSH, Joel;MIHALIC, Jeffrey;WU, Jeffrey;KATO, Daisuke;WEISS, Dahlia;PENG, Ge WO2020/81450, 2020, A1 Location in patent:Paragraph 0673; 0674; 0675

124-38-9
134 suppliers
$175.00/23402

69321-60-4
206 suppliers
$11.00/1g

76006-33-2
321 suppliers
$10.00/5g
118-90-1
533 suppliers
$13.00/1g

76006-33-2
321 suppliers
$10.00/5g

118-90-1
533 suppliers
$13.00/1g

76006-33-2
321 suppliers
$10.00/5g
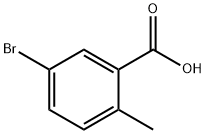
79669-49-1
445 suppliers
$5.00/1g
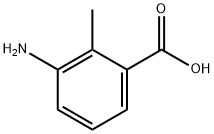
52130-17-3
335 suppliers
$20.00/25g

76006-33-2
321 suppliers
$10.00/5g