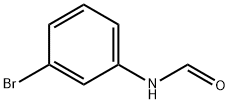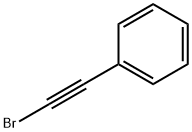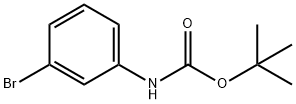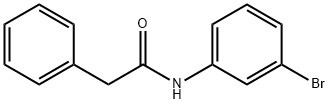
3'-Bromo-2-phenylacetoanilide synthesis
- Product Name:3'-Bromo-2-phenylacetoanilide
- CAS Number:13140-73-3
- Molecular formula:C14H12BrNO
- Molecular Weight:290.16
Yield:13140-73-3 66%
Reaction Conditions:
with bis(acetylacetonate)nickel(II);di-tert-butyl peroxide;carbon monoxide at 110; under 15001.5 Torr; for 16 h;Autoclave;
Steps:
(1) Typical procedure for the reaction of alkanes (1) and formanilides (2) without auxiliary solvent
General procedure: To a 50 mL of autoclave, was added cyclohexane (1a, 20 mL),formanilide (2a, 10 mmol), Ni(acac)2 (1 mmol, 0.26 g) and DTBP(12 mmol, 1.76 g). After replaced with argon twice, the autoclave was rinsed three times with CO and then heated to 110°C under 20 bar CO for 16 h. The autoclave is then cooled to room temperature and carefully relieved of pressure. The solid catalyst was filtered off. The excess alkane was removed under reduced pressureand the residue was separated by silica gel column chromatography (Petroleum ether/ethyl acetate) to give the product 3aa.
References:
Han, Zhang;Chaowei, Dai;Lice, Liu;Hongfei, Ma;Hongzhong, Bu;Yufeng, Li [Tetrahedron,2018,vol. 74,# 27,p. 3712 - 3718]