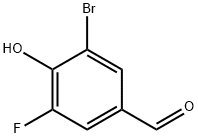
3-broMo-5-fluoro-4-hydroxybenzaldehyde synthesis
- Product Name:3-broMo-5-fluoro-4-hydroxybenzaldehyde
- CAS Number:185345-46-4
- Molecular formula:C7H4BrFO2
- Molecular Weight:219.01
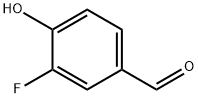
405-05-0

185345-46-4
Synthesis of 3-bromo-5-fluoro-4-hydroxybenzaldehyde (26): 3-fluoro-4-hydroxybenzaldehyde (2 g, 14.3 mmol, 1 eq.) was dissolved in acetic acid (60 mL) and bromine (2.7 g, 17.0 mmol, 1.2 eq.) was added slowly to a solution of acetic acid (10 mL). The reaction mixture was stirred at 45°C for 26 hours. After completion of the reaction, the solvent was removed by concentration under reduced pressure and saturated saline (50 mL) was added to the residue and extracted with ethyl acetate (3 x 80 mL). The organic layers were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was purified by reversed-phase semi-preparative high-performance liquid chromatography (HPLC) to afford the target product 3-bromo-5-fluoro-4-hydroxybenzaldehyde (26) 1.5 g in 48% yield.
1394291-51-0
24 suppliers
inquiry

185345-46-4
67 suppliers
$31.00/100mg
Yield:185345-46-4 88%
Reaction Conditions:
with oxygen;cobalt(II) diacetate tetrahydrate;sodium hydroxide in ethylene glycol at 80; under 760.051 Torr; for 8 h;
Steps:
General procedure
General procedure: a mixture of substrate 1a(1 mmol), cobalt salt (n1mol%) and NaOH (n2 equiv)in EG (5 mL) was stirred with O2 (1 atm) being bubbled, under 80 oCfor 8 h. Hydrochloric acid (10 mL, 2%) and methyl tert-butyl ether (MTBE, 10 mL) were successively added to the reactionmixture. The organic layer was separated, and the aqueous phase was furtherextracted with MTBE(10 mL × 2). The combined organic phase was dried over anhydrous sodium sulfateand concentrated to give a residue, which was purified by column chromatographyon silica gel (eluents: petroleum ether/ethyl acetate, 10/1) to provide thedesired products 2a.
References:
Jiang, Jian-An;Du, Jia-Lei;Liao, Dao-Hua;Wang, Zhan-Guo;Ji, Ya-Fei [Tetrahedron Letters,2014,vol. 55,# 8,p. 1406 - 1411] Location in patent:supporting information

405-05-0
237 suppliers
$12.00/1g

185345-46-4
67 suppliers
$31.00/100mg