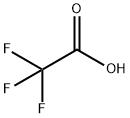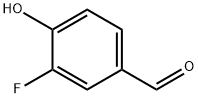
3-Fluoro-4-hydroxybenzaldehyde synthesis
- Product Name:3-Fluoro-4-hydroxybenzaldehyde
- CAS Number:405-05-0
- Molecular formula:C7H5FO2
- Molecular Weight:140.11
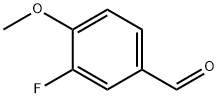
351-54-2

405-05-0
The general procedure for the synthesis of 3-fluoro-4-hydroxybenzaldehyde from 3-fluoro-4-methoxybenzaldehyde was as follows: 3-fluoro-4-methoxybenzaldehyde (1 g, 6.5 mmol) was mixed with boron tribromide (1 M solution in dichloromethane, 19.5 mmol) in dichloromethane, and the reaction was stirred for 24 hours under ice bath conditions. Upon completion of the reaction, an excess of anhydrous methanol was added to the reaction system, followed by removal of the solvent and the resulting trimethyl borate by distillation under reduced pressure. The residue was dissolved in methanol and again subjected to reduced pressure distillation to completely remove the residual trimethyl borate. The resulting solid residue was dissolved in ethyl acetate, the organic phase was washed with water (3 x 100 ml) and dried with anhydrous sodium sulfate. The organic solvent was removed by concentration under reduced pressure, and the crude product obtained was purified by silica gel column chromatography (eluent ratio of petroleum ether:ethyl ether=8:2) to finally obtain the pure 3-fluoro-4-hydroxybenzaldehyde. The yield was 91%; the melting point was 120-123 °C; the 1H NMR (CDCl3) data were as follows: δ 6.04 (s, exchangeable with D2O, 1H, OH); 7.16 (m, 1H, aromatic hydrogen); 7.62-7.67 (m, 2H, aromatic hydrogen); 9.86 (s, 1H, CHO). Elemental analysis results (C7H5FO2) Calculated values: C 60.01, H 3.60; measured values: C 59.93, H 3.77.

351-54-2
247 suppliers
$10.00/1g

405-05-0
237 suppliers
$12.00/1g
Yield:405-05-0 97%
Reaction Conditions:
with water;hydrogen bromide at 140; for 3 h;Inert atmosphere;
Steps:
3-Fluoro-4-hydroxybenzaldehyde (15)
3-Fluoro-4-methoxybenzaldehyde 14 (5.00 g, 32.5 mmol) was mixed with 48% (0088) HBr (30 ml_), heated to 140 °C and stirred under argon atmosphere for 3 h. The mixture was diluted with water (150 ml_) and extracted with dichloromethane (2x100 mL). The combined organic layers were washed with brine solution and dried over sodium sulfate. The solvent was removed in vacuo to give compound 15 as a brown solid (4.36 g, 30.2 mmol, 97% yield). NMRs are in accordance to literature. (Jiang 2014) ESI-MS (m/z): 141.00 [M+H]+.
References:
JULIUS-MAXIMILIANS-UNIVERSITAET WUERZBURG;CHEN, Xinyu;DECKER, Michael;HIGUCHI, Takahiro WO2020/148154, 2020, A1 Location in patent:Page/Page column 13; 14
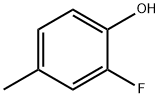
452-81-3
121 suppliers
$12.00/1g

405-05-0
237 suppliers
$12.00/1g
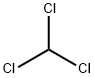
67-66-3
0 suppliers
$33.60/500ml
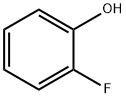
367-12-4
425 suppliers
$5.00/10g
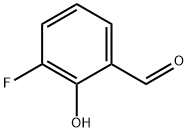
394-50-3
251 suppliers
$9.00/250mg

405-05-0
237 suppliers
$12.00/1g
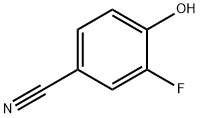
405-04-9
198 suppliers
$6.00/1g

405-05-0
237 suppliers
$12.00/1g