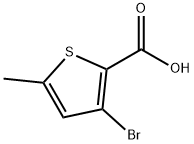
3-Bromo-5-methylthiophene-2-carboxylic acid synthesis
- Product Name:3-Bromo-5-methylthiophene-2-carboxylic acid
- CAS Number:61285-29-8
- Molecular formula:C6H5BrO2S
- Molecular Weight:221.07
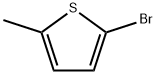
765-58-2
209 suppliers
$6.00/1g

124-38-9
134 suppliers
$214.00/14L

61285-29-8
30 suppliers
$305.00/100mg
Yield:61285-29-8 66%
Reaction Conditions:
Stage #1: 2-bromo-5-methyl thiophenewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -70; for 2 h;Inert atmosphere;
Stage #2: carbon dioxide in tetrahydrofuran;hexane at -70; for 1 h;Inert atmosphere;
Steps:
3-Bromo-5-methylthiophene-2-carboxylic acid (25, C6H5-BrO2S)
A 1.6 M solution of butyllithium in hexane(105 cm3) was slowly added at -30 C to a solution of18.4 g diisopropylamine (0.18 mol) in 150 cm3 oftetrahydrofuran. The mixture was stirred for 30 min at- 30 C, then cooled to - 70 C. At this temperature asolution of 24.8 g 2-bromo-5-methylthiophene (24,0.14 mol) in 60 cm3 of tetrahydrofuran was slowly added.The reaction mixture was stirred for 2 h at - 70 C, then astream of carbon dioxide was introduced at the sametemperature for 1 h. The addition of carbon dioxide wascontinued as the mixture warmed up to room temperature.The reaction mixture was acidified to pH 2 by addition of1 N hydrochloric acid and extracted with ethyl acetate. Theorganic layer was washed with water and brine, dried oversodium sulfate, and evaporated under reduced pressure.The remainder was purified by chromatography on silicagel, using ethyl acetate/heptane 1:3 as eluent, to deliver20.5 g 3-bromo-5-methylthiophene-2-carboxylic acid (25,66%). M.p.: 190-196 C; 1H NMR (400 MHz, DMSO-d6):d = 2.47 (s, 3H), 6.98 (s, 1H), 13.19 (bs, 1H) ppm; 13CNMR (100 MHz, CDCl3): d = 15.3, 108.7, 125.5, 129.8,141.4, 156.3 ppm; LC-MS: tR = 1.13 min; MS: m/z = 220, 222 ([M?1]?, [M?3]?).
References:
Walter, Harald;Lamberth, Clemens;Corsi, Camilla [Monatshefte fur Chemie,2018,vol. 149,# 4,p. 791 - 799]
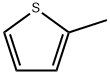
554-14-3
266 suppliers
$6.00/5g

61285-29-8
30 suppliers
$305.00/100mg