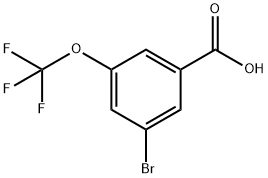
3-Bromo-5-(trifluoromethoxy)Benzoicacid synthesis
- Product Name:3-Bromo-5-(trifluoromethoxy)Benzoicacid
- CAS Number:453565-90-7
- Molecular formula:C8H4BrF3O3
- Molecular Weight:285.01
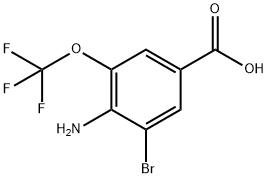
453565-89-4

453565-90-7
4-Amino-3-bromo-5-(trifluoromethoxy)benzoic acid (1.5 g, 5 mmol) was used as starting material, which was mixed with ethanol (15 mL) at 0 °C. Subsequently, concentrated sulfuric acid (2.26 g, 10.2 mmol) was slowly added. An aqueous solution (1.2 mL) of sodium nitrite (0.38 g, 5.5 mmol) was added dropwise at 0 °C and the dropwise process lasted for 1 hour. After the reaction mixture was gradually warmed to room temperature, it was heated to reflux for 45 min. Upon completion of the reaction, the reaction was terminated by the addition of water. The reaction mixture was extracted with dichloromethane and the organic phase was separated. The dichloromethane layer was dried and concentrated to give the crude product. The crude product was dissolved in 1M sodium hydroxide solution and extracted with ether to remove impurities. The aqueous phase was acidified with 2M HCl to pH=2 and precipitated as a solid, which was filtered and dried to give the target product 3-bromo-5-(trifluoromethoxy)benzoic acid (1.08 g, 75.5% yield).

453565-89-4
6 suppliers
inquiry

453565-90-7
106 suppliers
$25.00/1g
Yield: 75.5%
Reaction Conditions:
with sulfuric acid;sodium nitrite in sodium hydroxide;ethanol;water
Steps:
2 3-Bromo-5-trifluoromethoxybenzoic Acid
3-Bromo-5-trifluoromethoxybenzoic Acid 4-Amino-3-bromo-5-trifluoromethoxybenzoic acid (1.5 g, 5 mmoles) was mixed with ethanol (15 mL) at 0° C. and then concentrated sulfuric acid (2.26 g, 10.2 mmoles) wad added. The sodium nitrite (0.38 g, 5.5 mmoles) water solution (1.2 mL) was added dropwise at 0° C. for 1 hour. After the reaction mixture was warmed to room temperature and then heated to reflux for 45 minutes, water was added. The mixture was extracted with dichloromethane. The dichloromethane layer was dried and concentrated. The residue was dissolved in 1M sodium hydroxide and extracted with ether. The aqueous solution was acidified with 2M HCl to pH=2 to give 3-bromo-5-trifluoromethoxybenzoic acid (1.08 g, 75.5%).
References:
Wagenen, Bradford Van;Stormann, Thomas M.;Moe, Scott T.;Sheehan, Susan M.;McLeod, Donald A.;Smith, Daryl L.;Isaac, Methvin Benjamin;Slassi, Abdelmalik US2003/55085, 2003, A1