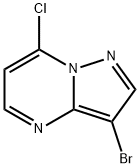
3-Bromo-7-chloropyrazolo[1,5-a]pyrimidine synthesis
- Product Name:3-Bromo-7-chloropyrazolo[1,5-a]pyrimidine
- CAS Number:877173-84-7
- Molecular formula:C6H3BrClN3
- Molecular Weight:232.47
![7-CHLOROPYRAZOLO[1,5-A]PYRIMIDINE](/CAS/20180808/GIF/58347-49-2.gif)
58347-49-2
![3-Bromo-7-chloropyrazolo[1,5-a]pyrimidine](/CAS/GIF/877173-84-7.gif)
877173-84-7
The general procedure for the synthesis of 3-bromo-7-chloropyrazolo[1,5-a]pyrimidines using 7-chloropyrazolo[1,5-a]pyrimidines as starting material was as follows: a solution of crude 7-chloropyrazolo[1,5-a]pyrimidines (44.2 g, 289 mmol) was cooled down to 0 °C in dichloromethane (289 mL) in the conditions of an ice bath. N-bromosuccinimide (51.4 g, 289 mmol) was slowly added, followed by stirring the reaction mixture for 16 hours at room temperature. After completion of the reaction, the dark orange reaction solution was diluted with dichloromethane (200 mL) and washed sequentially with 10% potassium carbonate solution (3 x 150 mL) and brine (100 mL). The organic phase was separated, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give a dark orange solid. The solid was ground with methanol to afford the target product 3-bromo-7-chloropyrazolo[1,5-a]pyrimidine (50.9 g, 76% yield) as a yellow solid. The product was analyzed by HPLC with a purity of 96%; 1H NMR (250 MHz, CDCl3) δ 8.49 (d, J = 5.2 Hz, 1H), 8.25 (s, 1H), 7.07 (d, J = 5.2 Hz, 1H); 13C NMR (62.9 MHz, DMSO) δ 150.4, 145.9, 144.7, 138.5. 109.5, 84.7.
![7-CHLOROPYRAZOLO[1,5-A]PYRIMIDINE](/CAS/20180808/GIF/58347-49-2.gif)
58347-49-2
153 suppliers
$23.00/250mg
![3-Bromo-7-chloropyrazolo[1,5-a]pyrimidine](/CAS/GIF/877173-84-7.gif)
877173-84-7
70 suppliers
$55.00/50mg
Yield:877173-84-7 91%
Reaction Conditions:
with N-Bromosuccinimide in acetonitrile at 20; for 10 h;
References:
Khirsariya, Prashant;Pospí?il, Patrik;Maier, Luká?;Boudny, Miroslav;Babá?, Martin;Kroutil, Ond?ej;Mráz, Marek;Vácha, Robert;Paruch, Kamil [Journal of Medicinal Chemistry,2022,vol. 65,# 7,p. 5701 - 5723]
![4H-PYRAZOLO[1,5-A]PYRIMIDIN-7-ONE](/CAS/GIF/29274-23-5.gif)
29274-23-5
71 suppliers
$120.00/1g
![3-Bromo-7-chloropyrazolo[1,5-a]pyrimidine](/CAS/GIF/877173-84-7.gif)
877173-84-7
70 suppliers
$55.00/50mg
![ethyl 7-oxo-4,7-dihydropyrazolo[1,5-a]pyrimidine-6-carboxylate](/CAS2/GIF/29274-18-8.gif)
29274-18-8
43 suppliers
$25.00/100mg
![3-Bromo-7-chloropyrazolo[1,5-a]pyrimidine](/CAS/GIF/877173-84-7.gif)
877173-84-7
70 suppliers
$55.00/50mg
![7-Oxo-4,7-dihydropyrazolo[1,5-a]pyrimidine-6-carboxylic acid](/CAS/GIF/197367-75-2.gif)
197367-75-2
27 suppliers
$220.00/500mg
![3-Bromo-7-chloropyrazolo[1,5-a]pyrimidine](/CAS/GIF/877173-84-7.gif)
877173-84-7
70 suppliers
$55.00/50mg