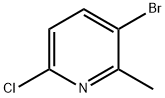
3-Bromo-6-chloro-2-methylpyridine synthesis
- Product Name:3-Bromo-6-chloro-2-methylpyridine
- CAS Number:132606-40-7
- Molecular formula:C6H5BrClN
- Molecular Weight:206.47
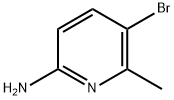
42753-71-9

132606-40-7
General procedure for the synthesis of 5-bromo-2-chloro-6-methylpyridine from 2-amino-5-bromo-6-methylpyridine: dichloromethane (900 mL) was added to a 2L three-necked round-bottomed flask, followed by the addition of 2-amino-6-methyl-5-bromopyridine (74.23 g, 0.39 mol), pyridine hydrochloride (139 g, 1.2 mol), sodium nitrite (83.26 g, 1.2 mol) and cuprous chloride (3.76 g, 5% w/w relative to the feedstock). The reaction mixture was cooled to 0-10 °C in an ice water bath. Hydrochloric acid (4.5 mL, 6% v/w vs. feedstock) was added slowly and stirred at 0-10 °C for 30 min. The cooling bath was removed and the mixture was continued to stir at room temperature for 1 hour. Upon completion of the reaction, the reaction was quenched with saturated aqueous sodium bicarbonate (400 mL) and the organic and aqueous layers were separated and the aqueous layer was extracted with dichloromethane (100 mL). The organic layers were combined and concentrated to dryness. Hexane (750 mL) was added to the residue and stirred. The solid was collected by filtration, washed with hexane, and the filtrate was concentrated to dryness to afford the pure product 5-bromo-2-chloro-6-methylpyridine (61 g, 70% yield) in light yellow crystalline form.

42753-71-9
398 suppliers
$6.00/5g

132606-40-7
237 suppliers
$5.00/1g
Yield: 70%
Reaction Conditions:
Stage #1:5-bromo-6-methyl-pyridin-2-ylamine with hydrogenchloride;pyridine hydrochloride;copper(l) chloride;sodium nitrite in dichloromethane;water at 0 - 20; for 1.5 h;
Stage #2: with sodium hydrogencarbonate in dichloromethane;water
Steps:
To a 2L 3-neck RBF was added CH2Cl2 (900 mL) followed by 2-amino-6-methyl- 5-bromopyridine (74.23 g, 0.39 mol), pyridine-HCl (139 g, 1.2 mol), NaNO2 (83.26 g, 1.2 mol) and CuCl (3.76 g, 5% w/w to starting material). The mixture was cooled to 0-10 0C in an ice- water bath and cone. HCl (4.5 mL, 6% v/w to starting material) was added dropwise and the mixture stirred at 0-10 0C for 30 min. The cooling bath was removed and the mixture was stirred at rt for Ih. The reaction mixture was quenched with saturated aqueous NaHCO3 (400 mL), the layers separated and the aqueous layer was extracted with CH2Cl2 (100 mL). The combined organic layers were concentrated to dryness and hexanes (750 mL) was added to the residue under stirring. The solid was filtered and washed with hexane and the filtrate was concentrated to dryness to afford pure product as a light yellow crystalline solid (61 g, 70%).
References:
ANORMED INC. WO2007/22371, 2007, A2 Location in patent:Page/Page column 49
126717-59-7
245 suppliers
$6.00/1g

132606-40-7
237 suppliers
$5.00/1g
22280-60-0
331 suppliers
$10.00/1g

132606-40-7
237 suppliers
$5.00/1g
164666-68-6
240 suppliers
$9.00/1g

132606-40-7
237 suppliers
$5.00/1g