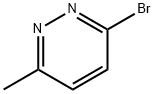
3-bromo-6-methylpyridazine synthesis
- Product Name:3-bromo-6-methylpyridazine
- CAS Number:65202-58-6
- Molecular formula:C5H5BrN2
- Molecular Weight:173.01
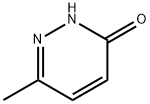
13327-27-0

65202-58-6
6-Methyl-3(2H)-pyridazinone (18 g, 163.44 mmol) was mixed with phosphorus tribromide oxide (93.8 g, 327.6 mmol) and the reaction was stirred at 70 °C overnight. Upon completion of the reaction, the reaction mixture was cooled to 40 °C and quenched by slowly pouring it into ice water. Subsequently, the reaction mixture was neutralized with saturated sodium bicarbonate solution (20 mL) and extracted with dichloromethane (100 mL x 3). The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to afford the target product 3-bromo-6-methylpyridazine (8 g, 31% yield). The product was detected by LCMS (ESI), m/z: 173 [M + 1]+. 1H NMR (chloroform-d, Bruker Avance 400MHz) data were as follows: δ 7.86-7.88 (d, 1H, J = 8Hz), 7.52-7.54 (d, 1H, J = 8Hz), 2.55 (s, 3H).

13327-27-0
227 suppliers
$9.00/1g

65202-58-6
114 suppliers
$16.00/250mg
Yield:65202-58-6 31%
Reaction Conditions:
with phosphorus(V) oxybromide at 70;
Steps:
13C Example 13C
A mixture of Example 13B (18g, 163.44mmol) and phosphorus oxybromide (93.8g, 327.6mmol) was stirred at70°C overnight. The mixture was cooled to 40°C, poured into ice-water, quenched with saturated sodium bicarbonate(20mL) and extracted with dichloromethane (100mL 3 3). The organic layers were combined, dried over anhydroussodium sulfate, filtered and evaporated to give the title compound, 3-bromo-6-methylpyridazine (8g, yield 31%). LCMS(ESI) m/z: 173[M+1]+. 1H NMR (CHLOROFORM-d, BrukerAvance 400MHz): ppm 7.86-7.88 (d, 1H, J = 8 Hz), 7.52-7.54(d, 1H, J = 8 Hz), 2.55(s, 3H).
References:
EP3333157,2018,A1 Location in patent:Paragraph 0136; 0137
157200-06-1
1 suppliers
inquiry

65202-58-6
114 suppliers
$16.00/250mg

539-88-8
374 suppliers
$5.00/5g

65202-58-6
114 suppliers
$16.00/250mg
94248-99-4
33 suppliers
$11.00/5g

65202-58-6
114 suppliers
$16.00/250mg
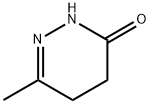
5157-08-4
147 suppliers
$11.00/1g

65202-58-6
114 suppliers
$16.00/250mg