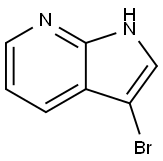
3-Bromo-7-azaindole synthesis
- Product Name:3-Bromo-7-azaindole
- CAS Number:74420-15-8
- Molecular formula:C7H5BrN2
- Molecular Weight:197.03
271-63-6

74420-15-8
General procedure for the synthesis of 3-bromo-1H-pyrrolo[2,3-b]pyridine from 7-azaindole: 100 mmol of 7-azaindole was dissolved in an appropriate amount of organic solvent and transferred to the reactor. Subsequently, 40 mmol of catalyst consisting of nickel(II) acetate mixed with triethanolamine borosilicate in a 3:1 ratio was added. The temperature of the reaction system was adjusted to 100 °C and the stirring speed was maintained at 90 rpm. The reaction was carried out by slowly passing 300 mmol of bromine gas and refluxed for 3 hours. After completion of the reaction, the target product 3-bromo-7-azaindole was obtained in 99.0% yield after purification.

271-63-6
573 suppliers
$9.00/5g

74420-15-8
254 suppliers
$8.00/1g
Yield: 99%
Reaction Conditions:
with 1-aza-4,6,11-trioxa-5-boratricyclo[3.3.3.0(1,5)]undecane;bromine;nickel diacetate at 100; for 3 h;Reflux;Temperature;
Steps:
6 Example 6
100mmol 7-azaindole was dissolved in organic solvent. Add into a reaction kettle. Add 40mmol catalyst. Maintain the temperature at 100 °C. 90 rpm/min of stirring. Place 300mmol bromine gas. Reflux reaction for 3h. Afterwards, completion of the reaction, purification to obtain 3-bromo-7-azaindole. Wherein the catalyst is nickel(II) acetate and triethanolamine boratemole ratio of 3:1 mixture. The yield of the product is 99.0%
References:
Ye, Fang CN106349242, 2017, A Location in patent:Paragraph 0033; 0034; 0035; 0036; 0037; 0038
![1H-PYRROLO[2,3-B]PYRIDINE-3-CARBOXYLIC ACID](/CAS/GIF/156270-06-3.gif)
156270-06-3
170 suppliers
$6.00/1g

74420-15-8
254 suppliers
$8.00/1g