Pyridine synthesis
- Product Name:Pyridine
- CAS Number:110-86-1
- Molecular formula:C5H5N
- Molecular Weight:79.1
Pyridine is produced from natural sources by Crowley Tar Products of Stow, Ohio, and Oklahoma City, Oklahoma (Harper et al. 1985; HSDB 1989; SRI 1986, 1987, 1988). Pyridine is synthetically produced by two companies, the Nepera Chemical Co. of Harriman, New York and the Reilly Tar and Chemical Corporation of Indianapolis, Indiana (Harper et al. 1985; SRI 1986, 1987, 1988).
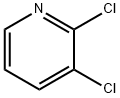
2402-77-9
673 suppliers
$5.00/5g

110-86-1
883 suppliers
$9.00/5g
Yield:110-86-1 100 %Spectr.
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen;sodium hydrogencarbonate in methanol at 20; under 760.051 Torr; for 2 h;Sealed tube;chemoselective reaction;
Steps:
General procedure forhydrodechlorination of 2-chloropyridine derivatives
General procedure: To a solution of the 2-chloropyridine derivative (1 equiv.) in MeOH (10 mL) was added 10% Pd/C (1 mol%) and NaHCO3 (1 equiv. per pyridine chlorine).The reaction vessel was sealed and flushed with hydrogen gas three times. The black suspension was stirred at room temperature under an atmosphere of hydrogen (1 atm.) for two hours. The mixture was filtered through celite washing with methanol and concentrated in vacuo. Purification by column chromatography was performed as required to yield the hydrodechlorinated pyridine product.
References:
Kinarivala, Nihar;Trippier, Paul C. [Tetrahedron Letters,2014,vol. 55,# 39,p. 5386 - 5389] Location in patent:supporting information
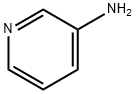
462-08-8
473 suppliers
$14.00/25g

110-86-1
883 suppliers
$9.00/5g
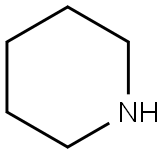
110-89-4
0 suppliers
$15.96/100ML

110-86-1
883 suppliers
$9.00/5g
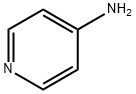
504-24-5
482 suppliers
$10.00/1g

110-86-1
883 suppliers
$9.00/5g