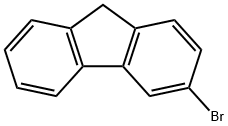
3-Bromo-9H-fluorene synthesis
- Product Name:3-Bromo-9H-fluorene
- CAS Number:2038-91-7
- Molecular formula:C13H9Br
- Molecular Weight:245.11
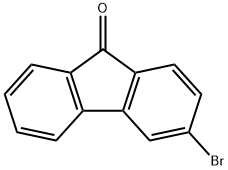
2041-19-2
111 suppliers
$16.00/250mg

2038-91-7
11 suppliers
inquiry
Yield:2038-91-7 98%
Reaction Conditions:
with hydrazine in ethanol;water;toluene for 2 h;Reflux;
Steps:
3-bromo-9H-fluorene
49 Ml (1000 mmol) of hydrazine hydrate and then 3 g of freshly prepared Raney nickel was added to a vigorously stirred refluxing suspension of 64 g (250 mmol) of 3-bromofluorene in a mixture of 1000 ml of toluene and 2000 ml of ethanol Add to water. After 2 hours at reflux, the mixture was cooled, the solvent was removed in vacuo and the residue taken up in 1000 ml of warmed chloroform, the solution was filtered through silica gel, the clear solution was evaporated to 100 ml and 300 Ml of ethanol is added. After allowing the mixture to stand for 12 hours, the colorless crystals were filtered off with suction and recrystallized twice from chloroform / ethanol. Yield: 60 g (240 mmol), 98% of theory; Purity: 97% according to 1 H-NMR.
References:
Merck Patent GmbH;Hussain, Farham Amir;Büsingen, Arne;Ploom, rzysztof;Mujika, FarnaudTeresa;STOESSEl, PHILIPP;Eberle, Thomas;Foges, Frank KR2017/59015, 2017, A Location in patent:Paragraph 0223; 0226-0228
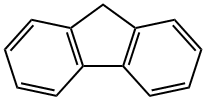
86-73-7
414 suppliers
$6.00/25g

2038-91-7
11 suppliers
inquiry
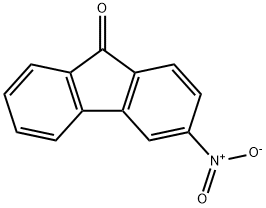
42135-22-8
21 suppliers
inquiry

2038-91-7
11 suppliers
inquiry
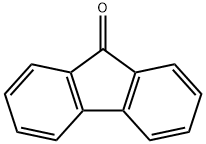
486-25-9
488 suppliers
$5.00/25g

2038-91-7
11 suppliers
inquiry
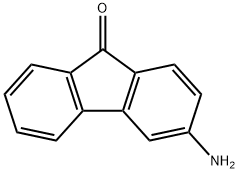
6276-05-7
11 suppliers
inquiry

2038-91-7
11 suppliers
inquiry