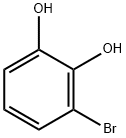
3-BROMOBENZENE-1,2-DIOL synthesis
- Product Name:3-BROMOBENZENE-1,2-DIOL
- CAS Number:14381-51-2
- Molecular formula:C6H5BrO2
- Molecular Weight:189.01
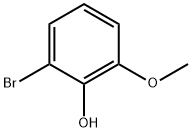
28165-49-3

14381-51-2
The general procedure for the synthesis of 3-bromocatechol from 2-bromo-6-methoxyphenol was as follows: to an anhydrous dichloromethane (40 mL) solution of 2-bromo-6-methoxyphenol (8.00 g, 39.4 mmol) was slowly added boron tribromide (1 M in dichloromethane, 43.1 mmol, 43.0 mL) at -78 °C. The reaction mixture was stirred for 1 hour at room temperature, then poured into ice water and continued to stir for 30 minutes. Subsequently, extraction was performed with dichloromethane (3 x 30 mL). The organic layers were combined, dried with anhydrous magnesium sulfate, and concentrated to give the target product 3-bromocatechol (6.90 g, 93% yield) as a brown oil. Thin layer chromatography (TLC) analysis showed an Rf value of 0.2 (unfolding agent: toluene/ethyl acetate = 9:1). The 1H NMR data of the product were as follows: δH 5.60 (1H, broad single peak, OH), 5.69 (1H, broad single peak, OH), 6.72 (1H, double peak, J=8.1 Hz), 6.87 (1H, double peak, J=1.5,8.1 Hz), 7.00 (1H, double peak, J=1.5,8.1 Hz); 13C NMR data: δC 109.5, 114.9, 121.9, 123.3, 140.3, 144.5.The 1H NMR spectra of the products were in agreement with the literature reports.

28165-49-3
112 suppliers
$5.00/250mg

14381-51-2
161 suppliers
$11.00/1g
Yield:14381-51-2 93%
Reaction Conditions:
Stage #1:6-bromoguaiacol with boron tribromide in dichloromethane at -78 - 20; for 1 h;
Stage #2: with water in dichloromethane for 0.5 h;Cooling with ice;
Steps:
4.2.17. 1-Bromo-2,3-dihydroxybenzene (3-bromocatechol)
To a cold (-78 °C) solution of 2-bromo-6-methoxyphenol (8.00 g, 39.4 mmol) in dry CH2Cl2 (40 mL) was added slowly BBr3 (1 M in CH2Cl2, 43.1 mmol, 43.0 mL). After stirring at rt for 1 h, the mixture was poured onto ice-water, stirred for 30 min and extracted with CH2Cl2 (3×30 mL). The organic layer was dried (MgSO4) then concentrated affording the title product as a brownish oil (6.90 g, 93%); Rf (toluene/EtOAc 9:1) 0.2; δH 5.60 (1H, br s, OH), 5.69 (1H, br s, OH), 6.72 (1H, dd, J 8.1, 8.1 Hz), 6.87 (1H, dd, J 1.5, 8.1 Hz), 7.00 (1H, dd, J 1.5, 8.1 Hz); δC 109.5, 114.9, 121.9, 123.3, 140.3, 144.5. 1H NMR is in accordance with the literature data.20
References:
Stephan, Michel;Zupancic, Borut;Mohar, Barbara [Tetrahedron,2011,vol. 67,# 34,p. 6308 - 6315] Location in patent:experimental part
5424-43-1
115 suppliers
$13.00/100mg

14381-51-2
161 suppliers
$11.00/1g
1829-34-1
255 suppliers
$33.00/1g

14381-51-2
161 suppliers
$11.00/1g
120-80-9
532 suppliers
$13.00/25g

14381-51-2
161 suppliers
$11.00/1g

108-86-1
523 suppliers
$10.00/5g

14381-51-2
161 suppliers
$11.00/1g