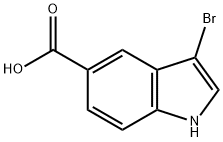
3-Bromoindole-5-carboxylic Acid synthesis
- Product Name:3-Bromoindole-5-carboxylic Acid
- CAS Number:916179-87-8
- Molecular formula:C9H6BrNO2
- Molecular Weight:240.05
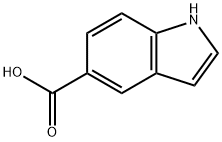
1670-81-1

916179-87-8
General procedure for the synthesis of 3-bromoindole-5-carboxylic acid from indole-5-carboxylic acid: to a solution of indole-5-carboxylic acid (1.0 g, 6.2 mmol) in N,N-dimethylformamide (DMF, 10 mL) was added bromine (Br2, 334 μL, 1.05 eq.) and the reaction was carried out at room temperature (see Scheme 9). After the reaction was complete (about 5 min), the reaction mixture was poured into ice-cold sodium sulfite (Na2SO3, 1% aqueous solution, 100 mL) to precipitate the product. The precipitate was collected by filtration and dried under high vacuum. The resulting product was a light beige solid (1.46 g, 98% yield) and could be used in subsequent reactions without further purification. The structure of the product was confirmed by 1H NMR (DMSO-d6): δ 7.47 (d, 1H, J = 8.6 Hz), 7.65 (d, 1H, J = 2.5 Hz), 7.76 (dd, 1H, J = 7.3, 1.3 Hz), 8.04 (s, 1H), 11.77 (s, 1H), 12.57 (s, 1H).

1670-81-1
342 suppliers
$6.00/250mg

916179-87-8
53 suppliers
$75.00/1g
Yield:916179-87-8 98%
Reaction Conditions:
with bromine in N,N-dimethyl-formamide at 20; for 0.0833333 h;
Steps:
6.i
To a solution of commercially available indole-5-carboxylic acid (1.0 g, 6.2 mmol) in DMF (10 mL) was added Br∑ (334 μL, 1.05 eq.) at room temperature EPO
References:
WO2006/125324,2006,A1 Location in patent:Page/Page column 94-95