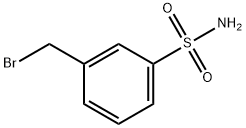
3-Bromomethylbenzenesulfonamide synthesis
- Product Name:3-Bromomethylbenzenesulfonamide
- CAS Number:220798-52-7
- Molecular formula:C7H8BrNO2S
- Molecular Weight:250.11
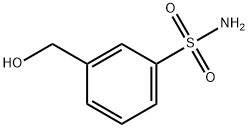
220798-42-5

220798-52-7
The general procedure for the synthesis of 3-(bromomethyl)benzene-1-sulfonamide from 3-(hydroxymethyl)benzene-1-sulfonamide was as follows: 1.48 g (7.90 mmol) of 3-(hydroxymethyl)benzene-sulfonamide was suspended in 25 mL of dichloromethane, and 2.35 g (8.69 mmol) of phosphorus tribromide was slowly added at 20°C. The reaction mixture was stirred overnight at room temperature. The reaction mixture was stirred overnight at room temperature. Upon completion of the reaction, the reaction was quenched by careful addition of water and the organic phase was separated. The aqueous phase was extracted twice with dichloromethane. All organic phases were combined, washed with saturated brine, dried over anhydrous magnesium sulfate and subsequently concentrated under reduced pressure. 1.9 g (94% yield) of 3-bromomethylbenzenesulfonamide crude product was obtained, which was pure enough to be used directly in the subsequent reaction.

220798-42-5
19 suppliers
inquiry

220798-52-7
74 suppliers
$51.00/100mg
Yield:220798-52-7 94%
Reaction Conditions:
with phosphorus tribromide in dichloromethane at 20;
Steps:
7 Preparation example 7Preparation of 3-(bromomethyl)benzenesulfonamide, (XII-1)
1.48 g (7.90 mmol)) 3-(hydroxymethyl)benzenesulfonamide were suspended in 25 ml dichloromethane and 2.35 g (8.69 mmol) phosphorus tribromide were added at 20 °C. The mixture was stirred overnight. Water was added carefully and the organic phase separated. The aqueous phase was two times extracted with dichloromethane. The combined organic phases were washed with brine, dried over magnesium sulfate and concentrated in vacuo.Yield: 1.9 g (94 %) 3-(bromomethyl)benzenesulfonamide as raw product, which was pure enough for the next step.
References:
WO2015/7668,2015,A1 Location in patent:Page/Page column 144
4025-64-3
216 suppliers
$6.00/1g

220798-52-7
74 suppliers
$51.00/100mg
636-76-0
90 suppliers
$10.00/100mg

220798-52-7
74 suppliers
$51.00/100mg
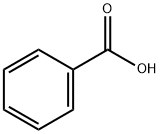
65-85-0
1441 suppliers
$10.00/25g

220798-52-7
74 suppliers
$51.00/100mg