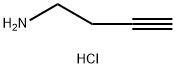
3-BUTYN-1-AMINE HYDROCHLORIDE synthesis
- Product Name:3-BUTYN-1-AMINE HYDROCHLORIDE
- CAS Number:88211-50-1
- Molecular formula:C4H8ClN
- Molecular Weight:105.57
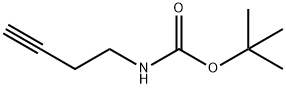
149990-27-2

88211-50-1
General procedure for the synthesis of 3-butyn-1-amine hydrochloride from N-Boc-butyn-4-amine: 1. N-Boc-butyn-4-amine (1.81 g, 0.1 mmol) was dissolved in dichloromethane (5 mL) and 5N aqueous hydrochloric acid solution (5 mL) was added. The reaction mixture was stirred vigorously at room temperature overnight. Upon completion of the reaction, the mixture was concentrated in vacuum to a minimum volume and then freeze-dried to afford 3-butyn-1-amine hydrochloride as a white solid (809 mg, 79% yield). Steps for the synthesis of N-tert-butyl-3-alkynyl-2,2-dimethylpropionamide: 1. Triethylamine (3 mL) was added to a suspension of 3-butyn-1-amine hydrochloride (200 mg, 2.1 mmol) in dichloromethane (10 mL) and stirred for 10 min at room temperature and under nitrogen protection. Pivaloyl chloride (284.9 μL, 2.31 mmol) was then added and stirring was continued overnight at room temperature and under nitrogen protection. After completion of the reaction, the reaction mixture was concentrated in vacuum and the residue was dissolved in methanol, filtered through a SCX-2 column and eluted with methanol to afford N-tert-butyl-3-ynyl-2,2-dimethylpropionamide (265 mg, 65% yield).

149990-27-2
103 suppliers
$38.00/250mg

88211-50-1
137 suppliers
$21.00/250mg
Yield:88211-50-1 79%
Reaction Conditions:
with hydrogenchloride;water in dichloromethane at 20;
Steps:
Preparation 18
Preparation 18; iV-But-3-ynyl-2,2-dimethyl-ρropionamide; But-3-ynyl amine hydrochloride: Dissolve but-3-ynyl-carbamic acid tert-butyl ester (1.81 g, 0.1 mmol) in DCM (5 mL) and add 5N aqueous HCl (5 mL). Stir vigorously at room temperature overnight. Concentrate in vacuo to a minimum amount of volume and then freeze dry to obtain the desired material as a white solid (809 mg, 79%).iV-But-3-ynyl-2,2-dimethγl-propionamide:; Add triethylamine (3 mL) to a suspension of but-3-ynylamine hydrochloride (200 mg, 2.1 mmol) in DCM (10 mL) and stir for 10 min at room temperature under nitrogen. Add then neat pivaloyl chloride (284.9 μL, 2.31 mmol) and stir at room temperature overnight under nitrogen. Concentrate in vacuo, take up the residue in methanol and filter through a SCX-2 cartridge eluting with methanol to obtain the title compound (265 mg, 65%).
References:
ELI LILLY AND COMPANY WO2007/28131, 2007, A1 Location in patent:Page/Page column 50
14396-90-8
74 suppliers
$57.00/2 g

88211-50-1
137 suppliers
$21.00/250mg

927-74-2
426 suppliers
$6.00/5g

88211-50-1
137 suppliers
$21.00/250mg

14396-90-8
74 suppliers
$57.00/2 g
1875-48-5
151 suppliers
$17.67/1gm:

88211-50-1
137 suppliers
$21.00/250mg
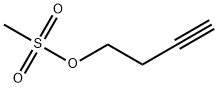
72486-09-0
36 suppliers
inquiry

88211-50-1
137 suppliers
$21.00/250mg