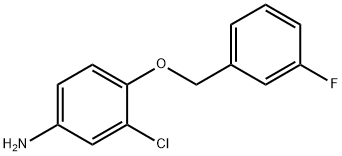
3-Chloro-4-(3-fluorobenzyloxy)aniline synthesis
- Product Name:3-Chloro-4-(3-fluorobenzyloxy)aniline
- CAS Number:202197-26-0
- Molecular formula:C13H11ClFNO
- Molecular Weight:251.68
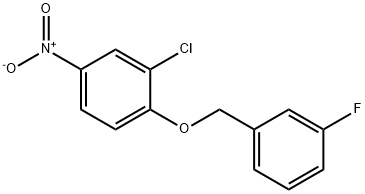
443882-99-3

202197-26-0
Example 5: Preparation of 3-chloro-4-(3-fluorobenzyloxy)aniline In a 1 L reactor, 35.6 g of Intermediate-A (3-chloro-4-(3-fluorobenzyloxy)nitrobenzene), 21.2 g of iron powder (70 mesh), and 60.9 g of ammonium chloride were dissolved in a solvent mixture of 506 mL of ethanol and 128 mL of water, and the reaction was carried out at reflux for 2 hours. Upon completion of the reaction, the reaction mixture was cooled to 20-25 °C and the insoluble iron oxide was removed by vacuum filtration. The solid obtained by filtration was washed with ethanol. The filtrates were combined and the solvent was removed by evaporation to give 94.9 g of a highly moist orange solid. The solid was mixed with 400 mL of dichloromethane to dissolve the organic product. The turbid solution was filtered to remove inorganic salts. The filtrate was transferred to a partition funnel to separate the organic phase and remove the aqueous residue. The organic phase was dried over anhydrous sodium sulfate and evaporated to dryness to give 30.8 g of 3-chloro-4-(3-fluorobenzyloxy)aniline (Yield: 96%, Purity as determined by HPLC: 99.67%, Major impurity content: 0.10%).

443882-99-3
182 suppliers
$6.00/1g

202197-26-0
310 suppliers
$6.00/1g
Yield:202197-26-0 100%
Reaction Conditions:
with H2;Pt/C in ethanol
Steps:
1.d (d)
(d) Preparation of 4-(3-fluorobenzyloxy)-3-chloro Aniline Under nitrogen, a Parr Shaker flask was charged with Pt/C 5% (135 mg) and ethanol (180 mL) and 4-(3-fluorobenzyloxy)-3-chloro nitrobenzene (13.5 g) was added. The reaction vessel was placed on a Parr Shaker Apparatus under 25 psi of H2 for 50 minutes. The catalyst was then removed by filtration through Celite and the filtrate concentrated to afford a light grey solid. The filtrate was triturated with diethyl ether and the solids collected by filtration (12.05 g, ~100% yield).
References:
Lackey, Karen Elizabeth;Spector, Neil;Wood lll, Edgar Raymond;Xia, Wenle US2004/53946, 2004, A1
3964-52-1
256 suppliers
$20.00/5g
456-41-7
297 suppliers
$13.00/25g

202197-26-0
310 suppliers
$6.00/1g

443882-99-3
182 suppliers
$6.00/1g
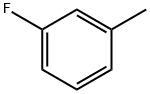
352-70-5
257 suppliers
$12.00/25mL

3964-52-1
256 suppliers
$20.00/5g

202197-26-0
310 suppliers
$6.00/1g
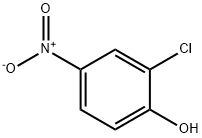
619-08-9
317 suppliers
$5.00/1g

202197-26-0
310 suppliers
$6.00/1g

456-41-7
297 suppliers
$13.00/25g

202197-26-0
310 suppliers
$6.00/1g