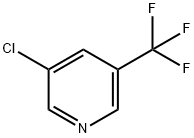
3-Chloro-5-(trifluoromethyl)pyridine synthesis
- Product Name:3-Chloro-5-(trifluoromethyl)pyridine
- CAS Number:85148-26-1
- Molecular formula:C6H3ClF3N
- Molecular Weight:181.54
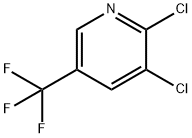
69045-84-7

85148-26-1
The general procedure for the synthesis of 3-chloro-5-trifluoromethylpyridine using 2,3-dichloro-5-trifluoromethylpyridine as starting material was as follows: zinc powder (1.18 g, 18.0 mmol) was added to a suspension of 2,3-dichloro-5-trifluoromethylpyridine (2.0 g, 9.30 mmol) in a solvent mixture of 80:20 (v/v) water and acetic acid (5 mL) at 90° C with stirring for 1 hour. Subsequently, zinc powder (1 g, 15.3 mmol) was made up and stirring was continued at 90°C for 15 minutes. After completion of the reaction, the mixture was cooled to room temperature, filtered and the filtrate was washed with dichloromethane. The filtrate was carefully concentrated and the residue was purified by silica gel column chromatography with an elution gradient of 100:0 to 0:100 (v/v) hexane:dichloromethane, resulting in 3-chloro-5-trifluoromethylpyridine as a colorless oil (0.120 g, 7% yield). The product was characterized by 1H NMR (300 MHz, CDCl3): δ 7.93 (s, 1H), 8.78 (s, 2H); GC-MS (EI) analysis showed m/z = 181.0, 183.0 [M].

69045-84-7
411 suppliers
$10.00/5g

85148-26-1
210 suppliers
$32.00/1g
Yield: 7%
Reaction Conditions:
with acetic acid;zinc in water at 90; for 1.25 h;
Steps:
7
Add zinc dust (1. 18 g) to a suspension of 2, 3-dichloro-5-trifluoromethyl-pyridine (2. 0 g, 9. 30 mmol) in 80 : 20 water and acetic acid (5 mL) and stir at 90 °C for 1 h. Add more zinc dust (1 g) and stir at 90 °C for an additional 15 min. Cool the reaction mixture to room temperature, filter, and wash with dichloromethane. Carefully concentrate and purify the residue by silica gel chromatography, eluting with 100 : 0 to 0 : 100 hexane : dichloromethane, to obtain the title compound as a volatile oil (0. 120g, 7%). 1H NMR (300 MHz, CDCl3) 8 7. 93 (s, 1 H), 8. 78 (s, 2 H). GC-MS (EI) m/z = 181. 0, 183. 0 [M].
References:
ELI LILLY AND COMPANY WO2005/94822, 2005, A1 Location in patent:Page/Page column 28
80289-91-4
40 suppliers
inquiry
3796-23-4
232 suppliers
$7.00/1g

85148-26-1
210 suppliers
$32.00/1g
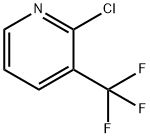
65753-47-1
377 suppliers
$8.00/5g
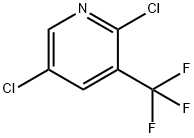
70158-59-7
90 suppliers
$31.00/100mg
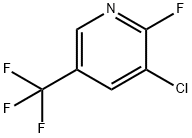
72537-17-8
196 suppliers
$11.00/1g

85148-26-1
210 suppliers
$32.00/1g
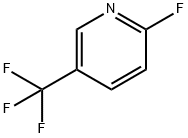
69045-82-5
210 suppliers
$5.00/1g