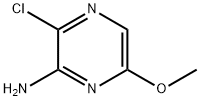
3-Chloro-6-Methoxypyrazin-2-aMine synthesis
- Product Name:3-Chloro-6-Methoxypyrazin-2-aMine
- CAS Number:13484-56-5
- Molecular formula:C5H6ClN3O
- Molecular Weight:159.57
6905-47-1

13484-56-5
General procedure for the synthesis of 2-amino-3-chloro-6-methoxypyrazine from 2-amino-6-methoxypyrazine: to a solution of 2-amino-6-methoxypyrazine (3 g, 24 mmol) in anhydrous N,N-dimethylformamide (DMF, 35 mL) was added N-chlorosuccinimide (NCS, 3.2 g, 24 mmol). The reaction mixture was stirred at room temperature for 16 h. After the reaction was completed, the mixture was poured into a mixture of ice and brine (130 mL). The aqueous phase was extracted with ethyl acetate (3 x 120 mL), the organic phases were combined, washed with 5% lithium chloride (LiCl) solution, dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure. The crude product was purified by SNAP-340-NH column chromatography (eluent: cyclohexane/ethyl acetate, gradient from 95:5 to 8:2) and SNAP1 00-Si-OH column chromatography (eluent: dichloromethane) to afford the target product, 2-amino-3-chloro-6-methoxypyrazine (1.98 g, 12.5 mmol, 52% yield). lc-MS (M-H) = 160.1.

6905-47-1
115 suppliers
$11.00/100mg

13484-56-5
26 suppliers
inquiry
Yield:13484-56-5 52%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at 20; for 16 h;
Steps:
1 Step 1 - Synthesis of 3-chloro-6-methoxypyrazin-2-amine:
To a solution of 6-methoxypyrazin-2-amine (3 g, 24 mmol) in dry DMF (35 mL), N-chlorosuccinimide (3.2 g, 24 mmol) was added. The reaction mixture was stirred at room temperature for 16 h then waspoured into ice and brine (130 mL). The aqueous solution was extracted with ethyl acetate (3 x 120 mL), the organic phase was washed with 5% solution of LiCI, dried over Na2SO4 and evaporated under reduced pressure. The crude was purified by SNAP-340-NH (cyclohexane/ethyl acetate from 95:5 up to 8:2) and by SNAP1 00-Si-OH (eluting with DCM)to afford the title intermediate (1.98 g, 12.5 mmol, 52% yield). LC-MS (M-H) = 160.1
References:
WO2017/211759,2017,A1 Location in patent:Page/Page column 84; 85