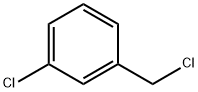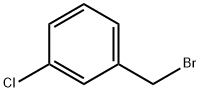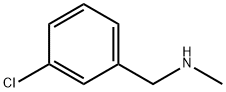
3-CHLORO-N-METHYLBENZYLAMINE synthesis
- Product Name:3-CHLORO-N-METHYLBENZYLAMINE
- CAS Number:39191-07-6
- Molecular formula:C8H10ClN
- Molecular Weight:155.62
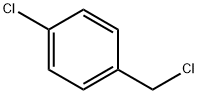
104-83-6

74-89-5

39191-07-6
GENERAL STEPS: An ethanol solution of methylamine (33% w/v, 17 mL) was slowly added dropwise to 4-chlorobenzyl chloride (2.0 g, 12.42 mmol), followed by heating the reaction mixture to reflux overnight. Upon completion of the reaction, ethanol was removed by distillation under reduced pressure by rotary evaporator. The residue was diluted with deionized water (100 mL) and washed with ethyl acetate (150 mL × 2). The aqueous phase was adjusted to pH 8 with solid potassium carbonate and then extracted with ethyl acetate (100 mL × 2). All organic phases were combined, washed with deionized water (100 mL), dried over anhydrous sodium sulfate, and finally the target product, N-methyl-3-chlorobenzylamine (1.7 g), was obtained by concentration under reduced pressure. The product was characterized by 1H NMR (300 MHz, DMSO-d6): δ 2.22 (s, 3H), 3.32 (br s, 1H), 3.62 (s, 2H), 7.25-7.38 (m, 4H); ESI-MS (m/z) 156 (M + H).
Yield: 1.7 g
Reaction Conditions:
in ethanolReflux;
Steps:
1 Step 1: 1 -(3 -Chlorophenyl)-N-methylmethanamine
An ethanolic solution of methylamine (33% w/v, 17 mL) was added to 4-chlorobenzyl chloride(2.0 g, 12.42 mmol) and the resulting mixture was refluxed overnight. Ethanol was removedby distillation. The residue was diluted with water (100 mL) and washed with ethyl acetate(150 mL x 2). The aqueous layer was basified with solid potassium carbonate till pH 8 andextracted with ethyl acetate (100 mL x 2). The combined organic layers were washed with water (100 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford 1.7 g of the titled compound. ‘H NMR (300 MHz, DMSO-d6) ö 2.22 (s, 3H), 3.32 (br s, 1H), 3.62 (s, 2H), 7.25-7.38 (m, 4H); ESI-MS (m/z) 156 (M+H).
References:
GLENMARK PHARMACEUTICALS S.A.;DAS, Sanjib;GHARAT, Laxmikant Atmaram;HARDE, Rajendra Laxman;THOMAS, Abraham;KHAIRATKAR-JOSHI, Neelima;SHAH, Daisy Manish;BAJPAI, Malini WO2017/199103, 2017, A1 Location in patent:Page/Page column 28
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

39191-07-6
93 suppliers
$17.00/1g

67-56-1
790 suppliers
$7.29/5ml-f
126799-85-7
3 suppliers
inquiry

39191-07-6
93 suppliers
$17.00/1g
15184-97-1
4 suppliers
inquiry