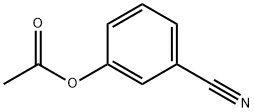
(3-cyanophenyl) acetate synthesis
- Product Name:(3-cyanophenyl) acetate
- CAS Number:55682-11-6
- Molecular formula:C9H7NO2
- Molecular Weight:161.16
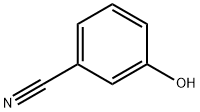
873-62-1
253 suppliers
$6.00/1g

55682-11-6
6 suppliers
$10.60/250mg
Yield:-
Reaction Conditions:
at 20; for 2 - 18 h;
Steps:
5
To a phenol, was added an acetylation reagent, such as acetyl chloride (1.0-2.0) or acetic anhydride, and the reaction mixture stirred for 2-18 hours at a temperature between room temperature and reflux. Excess acetylation reagent was removed in vacuo, then the O-acetylated phenol was treated with AICI3 (1.0-1.25 eq) and heated to a high temperature, such as 180° C for 30-60 minutes. The reaction mixture was then cooled, quenched with aqueous acid, and filtered to afford the desired ortho- ' hydroxyacetophenone.Hydroxyacetophenones were prepared, using the method of Hydroxyacetophenone Preparation 5, from commercially available reagents as follows: 4-Acetyl-3-hydroxy-benzonitrile from 3- hydroxybenzonitrile, 1-(5-chloro-4-fluoro-2-hydroxyphenyl)ethanone from 4-chloro-3-fluorophenol,
References:
PFIZER PRODUCTS INC. WO2008/65508, 2008, A1 Location in patent:Page/Page column 25-26
122-46-3
112 suppliers
$21.00/1g

55682-11-6
6 suppliers
$10.60/250mg

873-62-1
253 suppliers
$6.00/1g

108-24-7
5 suppliers
$14.00/250ML

55682-11-6
6 suppliers
$10.60/250mg

873-62-1
253 suppliers
$6.00/1g
830-03-5
182 suppliers
$10.00/5g
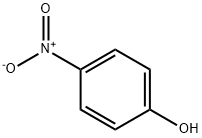
100-02-7
5 suppliers
$11.00/5G

55682-11-6
6 suppliers
$10.60/250mg
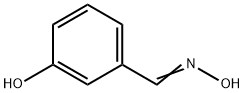
22241-18-5
8 suppliers
$110.00/50mg

108-24-7
5 suppliers
$14.00/250ML

55682-11-6
6 suppliers
$10.60/250mg